Ionic Liquids as Organocatalysts for Nucleophilic Fluorination: Concepts and Perspectives
- PMID: 36080470
- PMCID: PMC9457570
- DOI: 10.3390/molecules27175702
Ionic Liquids as Organocatalysts for Nucleophilic Fluorination: Concepts and Perspectives
Abstract
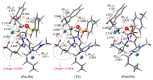
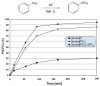
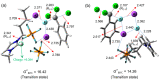
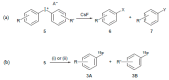
Besides their extremely useful properties as solvent, ionic liquids (ILs) are now considered to be highly instructive tools for enhancing the rates of chemical reactions. The ionic nature of the IL anion and cation seems to be the origin of this fascinating function of ILs as organocatalyst/promoter through their strong Coulombic forces on other ionic species in the reaction and also through the formation of hydrogen bonds with various functional groups in substrates. It is now possible to tailor-make ILs for specific purposes as solvent/promoters in a variety of situations by carefully monitoring these interactions. Despite the enormous potentiality, it seems that the application of ILs as organocatalysts/promoters for chemical reactions have not been fully achieved so far. Herein, we review recent developments of ILs for promoting the nucleophilic reactions, focusing on fluorination. Various aspects of the processes, such as organocatalytic capability, reaction mechanisms and salt effects, are discussed.
Keywords: ionic liquids; nucleophilic fluorination; organocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Harnessing Ionic Interactions and Hydrogen Bonding for Nucleophilic Fluorination.Molecules. 2020 Feb 7;25(3):721. doi: 10.3390/molecules25030721. Molecules. 2020. PMID: 32046021 Free PMC article. Review.
-
Dissolving Cellulose in 1,2,3-Triazolium- and Imidazolium-Based Ionic Liquids with Aromatic Anions.Molecules. 2020 Aug 2;25(15):3539. doi: 10.3390/molecules25153539. Molecules. 2020. PMID: 32748878 Free PMC article.
-
Enhancing the basicity of ionic liquids by tuning the cation-anion interaction strength and via the anion-tethered strategy.J Phys Chem B. 2014 Jan 30;118(4):1071-9. doi: 10.1021/jp4096503. Epub 2014 Jan 14. J Phys Chem B. 2014. PMID: 24387657
-
Facile and Environment-friendly Fluorinations using Ionic Liquids.Curr Org Synth. 2022;19(8):849-873. doi: 10.2174/1570179419666220208104453. Curr Org Synth. 2022. PMID: 35135452
-
Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives.Appl Microbiol Biotechnol. 2017 Jan;101(2):521-532. doi: 10.1007/s00253-016-8057-8. Epub 2016 Dec 24. Appl Microbiol Biotechnol. 2017. PMID: 28012046 Review.
Cited by
-
Advances in organocatalyzed synthesis of organic compounds.RSC Adv. 2024 Jun 25;14(28):20365-20389. doi: 10.1039/d4ra03046j. eCollection 2024 Jun 18. RSC Adv. 2024. PMID: 38919284 Free PMC article. Review.
-
Poly Caprolactam Supported Hexaethylene Glycolic Imidazolium Ionic Liquid as a Heterogeneous Promoter for Nucleophilic Fluorination.Molecules. 2023 Sep 21;28(18):6747. doi: 10.3390/molecules28186747. Molecules. 2023. PMID: 37764523 Free PMC article.
-
Contact ion-pair SN2 reactions activated by Lewis Base Phase transfer catalysts.Nat Commun. 2025 Jan 31;16(1):1236. doi: 10.1038/s41467-024-55795-6. Nat Commun. 2025. PMID: 39890785 Free PMC article.
References
-
- Yu L.H., editor. Ionic Liquids in Green Organic Synthesis and Catalysis. CRC press; New York, NY, USA: 2021.
-
- Sarkar A., Roy S.R., Parikh N., Chakraborti A.K. Nonsolvent Application of Ionic Liquids: Organo-Catalysis by 1-Alkyl-3-Methylimidazolium Cation Based Room-Temperature Ionic Liquids for Chemoselective N-Tert-Butyloxycarbonylation of Amines and the Influence of the C-2 Hydrogen on Catalytic Efficiency. J. Org. Chem. 2011;76:7132–7140. doi: 10.1021/jo201102q. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

