Computer-Aided Drug Design Boosts RAS Inhibitor Discovery
- PMID: 36080477
- PMCID: PMC9457765
- DOI: 10.3390/molecules27175710
Computer-Aided Drug Design Boosts RAS Inhibitor Discovery
Abstract
The Rat Sarcoma (RAS) family (NRAS, HRAS, and KRAS) is endowed with GTPase activity to regulate various signaling pathways in ubiquitous animal cells. As proto-oncogenes, RAS mutations can maintain activation, leading to the growth and proliferation of abnormal cells and the development of a variety of human cancers. For the fight against tumors, the discovery of RAS-targeted drugs is of high significance. On the one hand, the structural properties of the RAS protein make it difficult to find inhibitors specifically targeted to it. On the other hand, targeting other molecules in the RAS signaling pathway often leads to severe tissue toxicities due to the lack of disease specificity. However, computer-aided drug design (CADD) can help solve the above problems. As an interdisciplinary approach that combines computational biology with medicinal chemistry, CADD has brought a variety of advances and numerous benefits to drug design, such as the rapid identification of new targets and discovery of new drugs. Based on an overview of RAS features and the history of inhibitor discovery, this review provides insight into the application of mainstream CADD methods to RAS drug design.
Keywords: RAS inhibitor; computer-aided drug design; molecular docking; molecular dynamics simulation; virtual screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

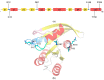
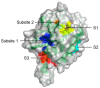

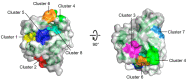
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

