Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA
- PMID: 36080497
- PMCID: PMC9457693
- DOI: 10.3390/molecules27175735
Extract of Acanthopanax senticosus and Its Components Interacting with Sulfide, Cysteine and Glutathione Increase Their Antioxidant Potencies and Inhibit Polysulfide-Induced Cleavage of Plasmid DNA
Abstract
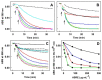
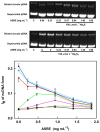
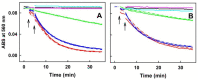
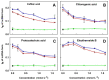
Aqueous root extract from Acanthopanax senticosus (ASRE) has a wide range of medicinal effects. The present work was aimed at studying the influence of sulfide, cysteine and glutathione on the antioxidant properties of ASRE and some of its selected phytochemical components. Reduction of the 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazol-1-yloxy-3-oxide (●cPTIO) stable radical and plasmid DNA (pDNA) cleavage in vitro assays were used to evaluate antioxidant and DNA-damaging properties of ASRE and its individual components. We found that the interaction of ASRE and its two components, caffeic acid and chlorogenic acid (but not protocatechuic acid and eleutheroside B or E), with H2S/HS-, cysteine or glutathione significantly increased the reduction of the ●cPTIO radical. In contrast, the potency of ASRE and its selected components was not affected by Na2S4, oxidized glutathione, cystine or methionine, indicating that the thiol group is a prerequisite for the promotion of the antioxidant effects. ASRE interacting with H2S/HS- or cysteine displayed a bell-shaped effect in the pDNA cleavage assay. However, ASRE and its components inhibited pDNA cleavage induced by polysulfides. In conclusion, we suggest that cysteine, glutathione and H2S/HS- increase antioxidant properties of ASRE and that changes of their concentrations and the thiol/disulfide ratio can influence the resulting biological effects of ASRE.
Keywords: Acanthopanax senticosus; DNA cleavage; H2S; antioxidant; caffeic acid; chlorogenic acid; eleutheroside; polysulfides; radical; ●cPTIO.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





Similar articles
-
Polysulfides and products of H2S/S-nitrosoglutathione in comparison to H2S, glutathione and antioxidant Trolox are potent scavengers of superoxide anion radical and produce hydroxyl radical by decomposition of H2O2.Nitric Oxide. 2018 Jun 1;76:136-151. doi: 10.1016/j.niox.2017.09.006. Epub 2017 Sep 23. Nitric Oxide. 2018. PMID: 28951200
-
The antioxidant activity and their major antioxidant compounds from Acanthopanax senticosus and A. koreanum.Molecules. 2015 Jul 22;20(7):13281-95. doi: 10.3390/molecules200713281. Molecules. 2015. PMID: 26205054 Free PMC article.
-
Acanthopanax senticosus Root Extract Exerts Dual Action on Mouse Ileal Smooth Muscle Function, Leading to Modulation of Gastrointestinal Motility.Biol Pharm Bull. 2020;43(5):817-822. doi: 10.1248/bpb.b19-01027. Biol Pharm Bull. 2020. PMID: 32378558
-
Acanthopanax senticosus: review of botany, chemistry and pharmacology.Pharmazie. 2011 Feb;66(2):83-97. Pharmazie. 2011. PMID: 21434569 Review.
-
Physiological role of hydrogen sulfide and polysulfide in the central nervous system.Neurochem Int. 2013 Nov;63(5):492-7. doi: 10.1016/j.neuint.2013.09.003. Epub 2013 Sep 11. Neurochem Int. 2013. PMID: 24036365 Review.
Cited by
-
Use of a rat model to characterize 35 arterial pulse wave parameters in a comparative study of isoflurane and Zoletil/xylazine anesthesia and the effect of Acanthopanax senticosus extract.Animal Model Exp Med. 2023 Oct;6(5):474-488. doi: 10.1002/ame2.12354. Epub 2023 Oct 12. Animal Model Exp Med. 2023. PMID: 37828718 Free PMC article.
References
-
- Li X., Chen C., Leng A., Qu J. Advances in the Extraction, Purification, Structural Characteristics and Biological Activities of Eleutherococcus senticosus Polysaccharides: A Promising Medicinal and Edible Resource with Development Value. Front. Pharmacol. 2021;12:753007. doi: 10.3389/fphar.2021.753007. - DOI - PMC - PubMed
-
- Vaško L., Vašková J., Fejerčáková A., Mojžišová G., Poráčová J. Comparison of some antioxidant properties of plant extracts from Origanum vulgare, Salvia officinalis, Eleutherococcus senticosus and Stevia rebaudiana. Vitr. Cell. Dev. Biol. Anim. 2014;50:614–622. doi: 10.1007/s11626-014-9751-4. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

