Use of magnetic resonance elastography to gauge meningioma intratumoral consistency and histotype
- PMID: 36081257
- PMCID: PMC9463601
- DOI: 10.1016/j.nicl.2022.103173
Use of magnetic resonance elastography to gauge meningioma intratumoral consistency and histotype
Abstract
Objective: To determine whether tumor shear stiffness, as measured by magnetic resonance elastography, corresponds with intratumoral consistency and histotype.
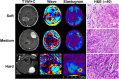
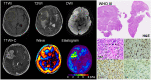
Materials and methods: A total of 88 patients with 89 meningiomas (grade 1, 74 typical [13 fibroblastic, 61 non-fibroblastic]; grade 2, 12 atypical; grade 3, 3 anaplastic) were prospectively studied, each undergoing preoperative MRE in conjunction with T1-, T2- and diffusion-weighted imaging. Contrast-enhanced T1-weighted sequences were also obtained. Tumor consistency was evaluated as heterogeneous or homogenous, and graded on a 5-point scale intraoperatively. MRE-determined shear stiffness was associated with tumor consistency by surgeon's evaluation and whole-slide histologic analyses.
Results: Mean tumor stiffness overall was 3.81+/-1.74 kPa (range, 1.57-12.60 kPa), correlating well with intraoperative scoring (r = 0.748; p = 0.001). MRE performed well as a gauge of tumor consistency (AUC = 0.879, 95 % CI: 0.792-0.938) and heterogeneity (AUC = 0.773, 95 % CI: 0.618-0.813), significantly surpassing conventional MR techniques (DeLong test, all p < 0.001 after Bonferroni adjustment). Shear stiffness was independently correlated with both fibrous content (partial correlation coefficient = 0.752; p < 0.001) and tumor cellularity (partial correlation coefficient = 0.547; p < 0.001). MRE outperformed other imaging techniques in distinguishing fibroblastic meningiomas from other histotypes (AUC = 0.835 vs 0.513 ∼ 0.634; all p < 0.05), but showed limited ability to differentiate atypical or anaplastic meningiomas from typical meningiomas (AUC = 0.723 vs 0.616 ∼ 0.775; all p > 0.05). Small (<2.5 cm, n = 6) and intraventricular (n = 2) tumors displayed inconsistencies between MRE and surgeon's evaluation.
Conclusions: The results of this prospective study provide substantial evidence that preoperative evaluation of meningiomas with MRE can reliably characterize tumor stiffness and spatial heterogeneity to aid neurosurgical planning.
Keywords: Elastography; Magnetic resonance imaging; Mechanical properties; Meningiomas; Stiffness.
Copyright © 2022. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The Mayo Clinic and RLE have intellectual property rights and a financial interest related to MRE technology.
Figures




References
-
- Cepeda S., Arrese I., Garcia-Garcia S., Velasco-Casares M., Escudero-Caro T., Zamora T., Sarabia R. Meningioma Consistency Can Be Defined by Combining the Radiomic Features of Magnetic Resonance Imaging and Ultrasound Elastography. A Pilot Study Using Machine Learning Classifiers. World Neurosurg. 2021;146:e1147–e1159. - PubMed
-
- Chakraborty A., Bamber J.C., Dorward N.L. Slip elastography: a novel method for visualising and characterizing adherence between two surfaces in contact. Ultrasonics. 2012;52(3):364–376. - PubMed
-
- Chen T.C., Zee C.S., Miller C.A., Weiss M.H., Tang G., Chin L., Levy M.L., Apuzzo M.L. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery. 1992;31:1015–1021. discussion 1021–1012. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

