Plaque-neutralizing antibody to BA.2.12.1, BA.4 and BA.5 in individuals with three doses of BioNTech or CoronaVac vaccines, natural infection and breakthrough infection
- PMID: 36081282
- PMCID: PMC9428331
- DOI: 10.1016/j.jcv.2022.105273
Plaque-neutralizing antibody to BA.2.12.1, BA.4 and BA.5 in individuals with three doses of BioNTech or CoronaVac vaccines, natural infection and breakthrough infection
Abstract
Background: BA.2.12.1, BA.4 and BA.5 subvariants of SARS-CoV-2 variant-of-concern (VOC) Omicron (B.1.1.529) are spreading globally. They demonstrate higher transmissibility and immune escape.
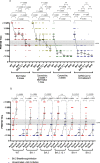
Objectives: Determine BA.2.12.1, BA.4 and BA.5 virus plaque reduction neutralization test (PRNT) antibody titres in individuals recently vaccinated with BNT162b2 (n = 20) or CoronaVac (n = 20) vaccines or those convalescent from ancestral wild- type (WT) SARS-CoV-2 (n = 20) or BA.2 infections with (n = 17) or without (n = 7) prior vaccination.
Results: Relative to neutralization of the WT virus, those vaccinated with BNT162b2 had 4.8, 3.4, 4.6, 11.3 and 15.5-fold reductions of geometric mean antibody titres (GMT) to BA.1, BA.2, BA.2.12.1, BA.4 and BA.5 viruses, respectively. Similarly, those vaccinated with CoronaVac had 8.0, 7.0, 11.8, 12.0 and 12.0 fold GMT reductions and those with two doses of CoronaVac boosted by BNT162b2 had 6.1, 6.7, 6,3, 13.0 and 21.2 fold GMT reductions to these viruses, respectively. Vaccinated individuals with BA.2 breakthrough infections had higher GMT antibody levels vs. BA.4 (36.9) and BA.5 (36.9) than unvaccinated individuals with BA.2 infections (BA.4 GMT 8.2; BA.5 GMT 11.0).
Conclusions: BA.4 and BA.5 subvariants were less susceptible to BNT162b2 or CoronaVac vaccine elicited antibody neutralization than subvariants BA.1, BA.2 and BA.2.12.1. Nevertheless, three doses BNT162b2 or booster of BNT162b2 following two doses of CoronaVac elicited detectable BA.4 and BA.5 neutralizing antibody responses while those vaccinated with three doses of CoronaVac largely fail to do so. BA.2 infections in vaccinated individuals led to higher levels of BA.4 or BA.5 neutralizing antibody compared to those who were vaccine-naive.
Keywords: COVID-19; Omicron, Neutralization; SARS-CoV-2; Vaccine.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest None of the authors had competing financial or non-financial interests.
Figures
References
-
- Altarawneh H.N., Chemaitelly H., Ayoub H., Hasan M.R., Coyle P., Yassine H.M., Al Khatib H.A., Benslimane F., Al-Kanaani Z., Al Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul Rahim H.F., Nasrallah G., Al Kuwari M.G., Butt A.A., Al Romaihi H.E., Al-Thani M.H., Al Khal A., Bertollini R., Tang P., Abu-Raddad L.J. Protection of SARS-CoV-2 natural infection against reinfection with the BA.4 or BA.5 Omicron subvariants. medRxiv. 2022 2022.2007.2011.22277448.
-
- Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., Al-Khatib H.A., Smatti M.K., Coyle P., Al-Kanaani Z., Al-Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul-Rahim H.F., Nasrallah G.K., Al-Kuwari M.G., Butt A.A., Al-Romaihi H.E., Al-Thani M.H., Al-Khal A., Bertollini R., Abu-Raddad L.J. Effects of previous infection and vaccination on symptomatic omicron infections. N. Engl. J. Med. 2022;387(1):21–34. - PMC - PubMed
-
- Bowen J.E., Addetia A., Dang H.V., Stewart C., Brown J.T., Sharkey W.K., Sprouse K.R., Walls A.C., Mazzitelli I.G., Logue J.K., Franko N.M., Czudnochowski N., Powell A.E., Dellota E., Jr., Ahmed K., Ansari A.S., Cameroni E., Gori A., Bandera A., Posavad C.M., Dan J.M., Zhang Z., Weiskopf D., Sette A., Crotty S., Iqbal N.T., Corti D., Geffner J., Snell G., Grifantini R., Chu H.Y., Veesler D. Omicron spike function and neutralizing activity elicited by a comprehensive panel of vaccines. Science. 2022;377(6608):890–894. - PMC - PubMed
-
- Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., Yu Y., Wang P., Zhang Z., Liu P., An R., Hao X., Wang Y., Wang J., Feng R., Sun H., Zhao L., Zhang W., Zhao D., Zheng J., Yu L., Li C., Zhang N., Wang R., Niu X., Yang S., Song X., Chai Y., Hu Y., Shi Y., Zheng L., Li Z., Gu Q., Shao F., Huang W., Jin R., Shen Z., Wang Y., Wang X., Xiao J., Xie X.S. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602. - PMC - PubMed
-
- Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., San J.E., Cromer D., Scheepers C., Amoako D.G., Karim F., Bernstein M., Lustig G., Archary D., Smith M., Ganga Y., Jule Z., Reedoy K., Hwa S.H., Giandhari J., Blackburn J.M., Gosnell B.I., Abdool Karim S.S., Hanekom W., von Gottberg A., Bhiman J.N., Lessells R.J., Moosa M.S., Davenport M.P., de Oliveira T., Moore P.L., Sigal A. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602(7898):654–656. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

