Psychosocial Impact of Demodex Blepharitis
- PMID: 36081601
- PMCID: PMC9447456
- DOI: 10.2147/OPTH.S374530
Psychosocial Impact of Demodex Blepharitis
Abstract
Purpose: To evaluate the impact of Demodex blepharitis on patients' daily activities and quality of life.
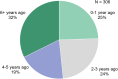
Patients and methods: In this multicenter, observational, prospective, IRB-approved study, 311 Demodex blepharitis patients aged ≥18 years were included. Demodex blepharitis was diagnosed based on the presence of ≥1.0 mite per lash (upper and lower eyelids combined), >10 collarettes on the upper lashes, and at least mild lid margin erythema of the upper eyelid in at least one eye. All patients were asked to complete a questionnaire about their symptoms, daily activities, quality of life, and management approaches, and descriptive statistics were used to analyze the responses.
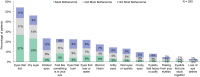
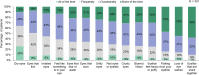
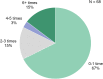
Results: More than half the patients had been experiencing symptoms of blepharitis for ≥4 years. The three most frequent and bothersome symptoms experienced by patients were dry eyes, itchiness, and irritation. Nearly half the patients (47%) responded that they were conscious of their eyes all day, and 23% said that they were constantly worrying about their eyes. Other activities that were negatively affected included difficulty driving at night (47%), additional time needed for daily hygiene routine (30%), and difficulty in wearing eye make-up (in 34% of females). While all subjects had objective signs of Demodex blepharitis confirmed by an eye care provider, 58% said they had never previously been diagnosed with blepharitis. The most commonly used management approaches for Demodex blepharitis were artificial tears (47%), warm compresses (32%), and lid wipes (14%).
Conclusion: Demodex blepharitis has a significant negative impact on daily activities and the mental and physical well-being of afflicted patients.
Keywords: Demodex blepharitis; Demodex mites; collarettes; cylindrical dandruff; dry eye disease; lid margin disease.
© 2022 O’Dell et al.
Conflict of interest statement
JG, EY and DKD have received consulting fees from Tarsus Pharmaceuticals. SNB and MH are employees of Tarsus Pharmaceuticals. DSD has received consulting and research fees from Tarsus Pharmaceuticals and honoraria from Azura. WOW reports personal fees from Tarsus Pharmaceuticals, during the conduct of the study; personal fees from Alcon, Bruder, Baush and Lomb, Azura Pharmaceuticals, Kala Pharmaceuticals, Oyster Point, Regenereyes, Santen, Sight Sciences, Sun Pharmaceuticals and Thea Pharmaceuticals, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures

References
-
- Lindstrom R, Donnenfeld E, Foulks G. Blepharitis update on research and management 2010. The New York Eye and Ear infirmary, MedEcus, Ophthalmolology Timesa Continuing Medical Education monograph; 2010.
LinkOut - more resources
Full Text Sources

