Oligomeropathies, inflammation and prion protein binding
- PMID: 36081661
- PMCID: PMC9445368
- DOI: 10.3389/fnins.2022.822420
Oligomeropathies, inflammation and prion protein binding
Abstract
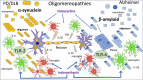
The central role of oligomers, small soluble aggregates of misfolded proteins, in the pathogenesis of neurodegenerative disorders is recognized in numerous experimental conditions and is compatible with clinical evidence. To underline this concept, some years ago we coined the term oligomeropathies to define the common mechanism of action of protein misfolding diseases like Alzheimer, Parkinson or prion diseases. Using simple experimental conditions, with direct application of synthetic β amyloid or α-synuclein oligomers intraventricularly at micromolar concentrations, we could detect differences and similarities in the biological consequences. The two oligomer species affected cognitive behavior, neuronal dysfunction and cerebral inflammatory reactions with distinct mechanisms. In these experimental conditions the proposed mediatory role of cellular prion protein in oligomer activities was not confirmed. Together with oligomers, inflammation at different levels can be important early in neurodegenerative disorders; both β amyloid and α-synuclein oligomers induce inflammation and its control strongly affects neuronal dysfunction. This review summarizes our studies with β-amyloid or α-synuclein oligomers, also considering the potential curative role of doxycycline, a well-known antibiotic with anti-amyloidogenic and anti-inflammatory activities. These actions are analyzed in terms of the therapeutic prospects.
Keywords: Alzheimer; Parkinson; amyloid; gliosis; neurotoxicity; oligomers.
Copyright © 2022 Forloni, La Vitola and Balducci.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aboushady Y., Gabr M., ElHady A. K., Salah M., Abadi A. H., Wilms G., et al. (2021). Discovery of hydroxybenzothiazole urea compounds as multitargeted agents suppressing major cytotoxic mechanisms in neurodegenerative diseases. ACS Chem. Neurosci. 12 4302–4318. 10.1021/acschemneuro.1c00475 - DOI - PubMed
-
- Acuña L., Hamadat S., Corbalán N. S., González-Lizárraga F., Dos-Santos-Pereira M., Rocca J., et al. (2019). Rifampicin and its derivative rifampicin quinone reduce microglial inflammatory responses and neurodegeneration induced in vitro by α-synuclein fibrillary aggregates. Cells 8:776. 10.3390/cells8080776 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

