The territorial nature of aggression in biofilms
- PMID: 36081784
- PMCID: PMC9445555
- DOI: 10.3389/fmicb.2022.878223
The territorial nature of aggression in biofilms
Abstract
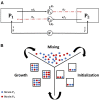
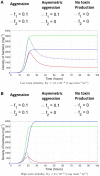
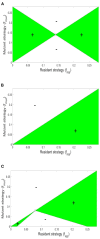
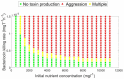
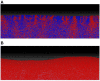
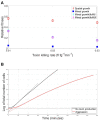
Microbial conflicts have a particularly aggressive nature. In addition to other chemical, mechanical, and biological weapons in their repertoire, bacteria have evolved bacteriocins, which are narrow-spectrum toxins that kill closely related strains. Bacterial cells are known to frequently use their arsenal while competing against each other for nutrients and space. This stands in contrast with the animal world, where conflicts over resources and mating opportunities are far less lethal, and get commonly resolved via ritualized fighting or "limited war" tactics. Prevalence of aggression in microbial communities is usually explained as due to their limited ability to resolve conflicts via signaling as well as their limited ability to pull out from conflicts due to the sessile nature of their life within biofilms. We use an approach that combines Evolutionary Game Theory (EGT) and Individual-based Modeling (IbM) to investigate the origins of aggression in microbial conflicts. In order to understand how the spatial mode of growth affects the cost of a fight, we compare the growth dynamics emerging from engaging in aggression in a well-mixed system to a spatially structured system. To this end, a mathematical model is constructed for the competition between two bacterial strains where each strain produces a diffusible toxin to which the other strain is sensitive. It is observed that in the biofilm growth mode, starting from a mixed layer of two strains, mutual aggression gives rise to an exceedingly high level of spatial segregation, which in turn reduces the cost of aggression on both strains compared to when the same competition occurs in a well-mixed culture. Another observation is that the transition from a mixed layer to segregated growth is characterized by a switch in the overall growth dynamics. An increased "lag time" is observed in the overall population growth curve that is associated with the earlier stages of growth, when each strain is still experiencing the inhibiting effect of the toxin produced by its competitor. Afterwards, an exponential phase of growth kicks in once the competing strains start segregating from each other. The emerging "lag time" arises from the spiteful interactions between the two strains rather than acclimation of cells' internal physiology. Our analysis highlights the territorial nature of microbial conflicts as the key driver to their elevated levels of aggression as it increases the benefit-to-cost ratio of participating in antagonistic interactions.
Keywords: aggression; bacteriocins; biofilms; evolutionary game theory (EGT); individual based modeling.
Copyright © 2022 Hashem and Van Impe.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Anderson D. A., Tannehill J. C., Pletcher R. H. (1997). Computational Fluid Mechanics and Heat Transfer. Boca Raton, FL: Taylor & Francis.
-
- Brännström Å., Johansson J., Von Festenberg N. (2013). The Hitchhiker's guide to adaptive dynamics. Games 4, 304–328. 10.3390/g4030304 - DOI
LinkOut - more resources
Full Text Sources

