Development of a loop-mediated isothermal amplification assay for the rapid detection of Russula subnigricans and Russula japonica
- PMID: 36081806
- PMCID: PMC9445624
- DOI: 10.3389/fmicb.2022.918651
Development of a loop-mediated isothermal amplification assay for the rapid detection of Russula subnigricans and Russula japonica
Abstract
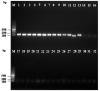
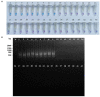
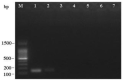
Russula subnigricans is the only deadly species in the genus Russula with a mortality rate of more than 50%, and Russula japonica is the most common poisonous species, making rapid species identification in mushroom poisoning incidents extremely important. The main objective of this study was to develop a rapid, specific, sensitive, and simple loop-mediated isothermal amplification (LAMP) assay for the detection of R. subnigricans and R. japonica. Two sets of species-specific LAMP primers targeting internal transcribed spacer (ITS) regions were designed to identify R. subnigricans and R. japonica. The results demonstrated that while LAMP could specifically detect R. subnigricans and R. japonica, the polymerase chain reaction (PCR) could not distinguish R. subnigricans from Russula nigricans. In addition, the results demonstrated that, compared to electrophoresis-LAMP and real-time quantitative LAMP (RT-qLAMP), the detection sensitivity of HNB-LAMP (a mixture of LAMP with hydroxy naphthol blue (HNB) dye) for R. subnigricans could reach 0.5 pg/μl and was 100-fold higher than that of PCR. The LAMP reaction could be completed in 45 min, which is much faster than the conventional PCR. In the future, LAMP can be used a quick, specific, and sensitive detection tool in various fields.
Keywords: ITS; Russula japonica; Russula subnigricans; loop-mediated isothermal amplification; mushroom poisoning.
Copyright © 2022 Long, Jiang, He and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Adamčík S., Looney B., Cabon M., Jančovičová S., Adamčíková K., Avis P. G., et al. (2019). The quest for a globally comprehensible Russula language. Fungal Divers. 99, 369–449. 10.1007/s13225-019-00437-2 - DOI
-
- Baek Y. H., Um J., Antigua K. J. C., Parket J., Kim Y., Oh S., et al. (2020). Development of a reverse transcription-loop-mediated isothermal amplification as a rapid early-detection method for novel SARS-CoV-2. Emerg. Microbes. Infect. 9, 998–1007. 10.1080/22221751.2020.1756698 - DOI - PMC - PubMed
-
- Barreda-García S., Miranda-Castro R., de-Los-Santos-Álvarez N., Miranda-Ordieres A. J., Lobo-Castañón M. J. (2018). Helicase-dependent isothermal amplification: a novel tool in the development of molecular-based analytical systems for rapid pathogen detection. Anal. Bioanal. Chem. 410, 679–693. 10.1007/s00216-017-0620-3 - DOI - PMC - PubMed
-
- Buyck B., Zoller S., Hofstetter V. (2018). Walking the thin line… ten years later: the dilemma of above- versus below-ground features to support phylogenies in the Russulaceae (Basidiomycota). Fungal Divers. 89, 267–292. 10.1007/s13225-018-0397-5 - DOI
-
- Chen G. F., Ma C. S., Zhang C. Y., Zhou J., Wang Y. Y., Wang G. C., et al. (2013). A rapid and sensitive method for field detection of Prorocentrum donghaiense using reverse transcription-coupled loop-mediated isothermal amplification. Harmful Algae 29, 31–39. 10.1016/j.hal.2013.08.001 - DOI
LinkOut - more resources
Full Text Sources

