Renovation as innovation: Repurposing human antibacterial peptide LL-37 for cancer therapy
- PMID: 36081952
- PMCID: PMC9445486
- DOI: 10.3389/fphar.2022.944147
Renovation as innovation: Repurposing human antibacterial peptide LL-37 for cancer therapy
Abstract
In many organisms, antimicrobial peptides (AMPs) display wide activities in innate host defense against microbial pathogens. Mammalian AMPs include the cathelicidin and defensin families. LL37 is the only one member of the cathelicidin family of host defense peptides expressed in humans. Since its discovery, it has become clear that they have pleiotropic effects. In addition to its antibacterial properties, many studies have shown that LL37 is also involved in a wide variety of biological activities, including tissue repair, inflammatory responses, hemotaxis, and chemokine induction. Moreover, recent studies suggest that LL37 exhibits the intricate and contradictory effects in promoting or inhibiting tumor growth. Indeed, an increasing amount of evidence suggests that human LL37 including its fragments and analogs shows anticancer effects on many kinds of cancer cell lines, although LL37 is also involved in cancer progression. Focusing on recent information, in this review, we explore and summarize how LL37 contributes to anticancer effect as well as discuss the strategies to enhance delivery of this peptide and selectivity for cancer cells.
Keywords: LL37; anticancer; antimicrobial peptides; cancer; cathelicidin (LL37); hCAP18.
Copyright © 2022 Lu, Zhu, Zhang and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor ZH declared a shared parent affiliation with the authors at the time of review.
Figures
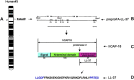



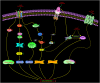
References
-
- Açil Y., Torz K., Gülses A., Wieker H., Gerle M., Purcz N., et al. (2018). An experimental study on antitumoral effects of KI-21-3, a synthetic fragment of antimicrobial peptide LL-37, on oral squamous cell carcinoma. J. Craniomaxillofac. Surg. 46 (9), 1586–1592. 10.1016/j.jcms.2018.05.048 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

