Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles
- PMID: 36082164
- PMCID: PMC9446449
- DOI: 10.3389/fbioe.2022.870193
Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles
Abstract
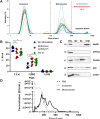
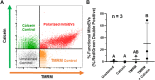
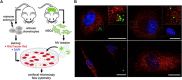
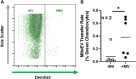
Cartilage and other skeletal soft tissues heal poorly after injury, in part due to their lack of vascularity and low metabolic rate. No pharmacologic approaches have proven effective in preventing chronic degenerative disease after joint injury. Mesenchymal stromal cells (MSCs) have been investigated for their ability to treat pain associated with osteoarthritis (OA) and preserve articular cartilage. Limitations of MSCs include variability in cell phenotype, low engraftment and retention rates, and inconsistent clinical outcomes. Therefore, acellular biologic therapies such as extracellular vesicles (EVs) are currently being investigated. MSC-derived EVs have been found to replicate many of the therapeutic effects of their cells of origin, but the mechanisms driving this remain unclear. Recent evidence in non-orthopedic tissues suggests MSCs can rescue injured cells by donating mitochondria, restoring mitochondrial function in recipient cells, preserving cell viability, and promoting tissue repair. Our group hypothesized that MSCs package mitochondria for export into EVs, and that these so-called "mitoEVs" could provide a delivery strategy for cell-free mitochondria-targeted therapy. Therefore, the goals of this study were to: 1) characterize the vesicle fractions of the MSCs secretome with respect to mitochondrial cargoes, 2) determine if MSC-EVs contain functional mitochondria, and 3) determine if chondrocytes can take up MSC-derived mitoEVs. We isolated exosome, microvesicle, and vesicle-free fractions from MSC-conditioned media. Using a combination of dynamic light scattering and nanoparticle tracking, we determined that MSC-EV populations fall within the three size categories typically used to classify EVs (exosomes, microvesicles, apoptotic bodies). Fluorescent nanoparticle tracking, immunoblotting, and flow cytometry revealed that mitochondrial cargoes are abundant across all EV size populations, and mitoEVs are nearly ubiquitous among the largest EVs. Polarization staining indicated a subset of mitoEVs contain functional mitochondria. Finally, flow cytometry and fluorescent imaging confirmed uptake of mitoEVs by chondrocytes undergoing rotenone/antimycin-induced mitochondrial dysfunction. These data indicate that MSCs package intact, functional mitochondria into EVs, which can be transferred to chondrocytes in the absence of direct cell-cell interactions. This work suggests intercellular transfer of healthy MT to chondrocytes could represent a new, acellular approach to augment mitochondrial content and function in poorly-healing avascular skeletal soft tissues.
Keywords: MSCs; mitoEVs; mitochondria; mitochondrial transfer; regenerative orthobiologic; secretome.
Copyright © 2022 Thomas, Fahey, Pugliese, Irwin, Antonyak and Delco.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mesenchymal stromal cells donate mitochondria to articular chondrocytes exposed to mitochondrial, environmental, and mechanical stress.Sci Rep. 2022 Dec 13;12(1):21525. doi: 10.1038/s41598-022-25844-5. Sci Rep. 2022. PMID: 36513773 Free PMC article.
-
Bone Marrow MSC Secretome Increases Equine Articular Chondrocyte Collagen Accumulation and Their Migratory Capacities.Int J Mol Sci. 2022 May 21;23(10):5795. doi: 10.3390/ijms23105795. Int J Mol Sci. 2022. PMID: 35628604 Free PMC article.
-
Human multipotent mesenchymal stromal cells cytokine priming promotes RAB27B-regulated secretion of small extracellular vesicles with immunomodulatory cargo.Stem Cell Res Ther. 2020 Dec 14;11(1):539. doi: 10.1186/s13287-020-02050-6. Stem Cell Res Ther. 2020. PMID: 33317598 Free PMC article.
-
The Potential Use of Mitochondrial Extracellular Vesicles as Biomarkers or Therapeutical Tools.Int J Mol Sci. 2023 Apr 10;24(8):7005. doi: 10.3390/ijms24087005. Int J Mol Sci. 2023. PMID: 37108168 Free PMC article. Review.
-
MitoEVs: A new player in multiple disease pathology and treatment.J Extracell Vesicles. 2023 Apr;12(4):e12320. doi: 10.1002/jev2.12320. J Extracell Vesicles. 2023. PMID: 37002588 Free PMC article. Review.
Cited by
-
The Role of Extracellular Vesicles in the Pathogenesis and Treatment of Rheumatoid Arthritis and Osteoarthritis.Cells. 2023 Nov 27;12(23):2716. doi: 10.3390/cells12232716. Cells. 2023. PMID: 38067147 Free PMC article. Review.
-
Advancing stroke therapy: innovative approaches with stem cell-derived extracellular vesicles.Cell Commun Signal. 2024 Jul 22;22(1):369. doi: 10.1186/s12964-024-01752-1. Cell Commun Signal. 2024. PMID: 39039539 Free PMC article. Review.
-
Immune system-related plasma extracellular vesicles in healthy aging.Front Immunol. 2024 Apr 3;15:1355380. doi: 10.3389/fimmu.2024.1355380. eCollection 2024. Front Immunol. 2024. PMID: 38633262 Free PMC article.
-
Extracellular Vesicles from Human Induced Pluripotent Stem Cells Exhibit a Unique MicroRNA and CircRNA Signature.Int J Biol Sci. 2024 Nov 22;20(15):6255-6278. doi: 10.7150/ijbs.100113. eCollection 2024. Int J Biol Sci. 2024. PMID: 39664576 Free PMC article.
-
Immune System-Related Plasma Pathogenic Extracellular Vesicle Subpopulations Predict Osteoarthritis Progression.Int J Mol Sci. 2024 Nov 21;25(23):12504. doi: 10.3390/ijms252312504. Int J Mol Sci. 2024. PMID: 39684216 Free PMC article.
References
-
- Bennett M. P., Vivancos-Koopman I., Seewald L., Wells K., Robinette T., Delco M. L., et al. (2019). Intercellular mitochondrial transfer from mesenchymal stem cells to stressed chondrocytes. Osteoarthr. Cartil. 27, S51–S52. 10.1016/j.joca.2019.02.074 - DOI
-
- Bianchi G., Sinigaglia L. (20122012). Chondrogenesis, joint formation, and cartilage metabolism. Arthritis Res. Ther. 142, A5. 10.1186/AR3712 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

