Early life stress exacerbates behavioural and neuronal alterations in adolescent male mice lacking methyl-CpG binding protein 2 (Mecp2)
- PMID: 36082308
- PMCID: PMC9447412
- DOI: 10.3389/fnbeh.2022.974692
Early life stress exacerbates behavioural and neuronal alterations in adolescent male mice lacking methyl-CpG binding protein 2 (Mecp2)
Abstract
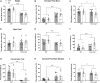
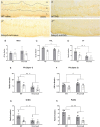
The methyl-CpG binding protein 2 gene (MECP2) encodes an epigenetic transcriptional regulator implicated in neuronal plasticity. Loss-of-function mutations in this gene are the primary cause of Rett syndrome and, to a lesser degree, of other neurodevelopmental disorders. Recently, we demonstrated that both Mecp2 haploinsuficiency and mild early life stress decrease anxiety-like behaviours and neuronal activation in brain areas controlling these responses in adolescent female mice. Here, we extend this work to males by using Mecp2-null and wild type adolescent mice subjected to maternal separation and their non-stressed controls. We assessed their behavioural responses in a battery of anxiety-provoking tests. Upon exposure to an elevated plus maze in aversive conditions, we evaluated changes in c-FOS expression in stress- and anxiety-related brain regions. In addition, we assessed the impact of maternal separation in neuronal maturation using doublecortin and reelin as surrogate markers. Mutant males showed reduced motor abilities, increased activation of the olfactory bulbs, probably due to breathing abnormalities, and decreased activation of the paraventricular thalamic nucleus, when compared to wild type mice. In addition, maternal separation increased the number of immature doublecortin-like neurons found in Mecp2-null animals. Moreover, this work shows for the first time that reelin is decreased in the mutant animals at the olfactory tubercle, piriform cortex and hippocampal dentate gyrus, an effect also associated to maternal separation. Taken together, our results suggest that maternal separation exacerbates some phenotypical alterations associated with lack of MeCP2 in adolescent males.
Keywords: Rett sydrome; c-FOS; doublecortin; maternal separation; reelin.
Copyright © 2022 Torres-Pérez, Martínez-Rodríguez, Forte, Blanco-Gómez, Stork, Lanuza, Santos and Agustín-Pavón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

