Structural and functional organization of the midline and intralaminar nuclei of the thalamus
- PMID: 36082310
- PMCID: PMC9445584
- DOI: 10.3389/fnbeh.2022.964644
Structural and functional organization of the midline and intralaminar nuclei of the thalamus
Abstract
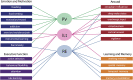
The midline and intralaminar nuclei of the thalamus form a major part of the "limbic thalamus;" that is, thalamic structures anatomically and functionally linked with the limbic forebrain. The midline nuclei consist of the paraventricular (PV) and paratenial nuclei, dorsally and the rhomboid and nucleus reuniens (RE), ventrally. The rostral intralaminar nuclei (ILt) consist of the central medial (CM), paracentral (PC) and central lateral (CL) nuclei. We presently concentrate on RE, PV, CM and CL nuclei of the thalamus. The nucleus reuniens receives a diverse array of input from limbic-related sites, and predominantly projects to the hippocampus and to "limbic" cortices. The RE participates in various cognitive functions including spatial working memory, executive functions (attention, behavioral flexibility) and affect/fear behavior. The PV receives significant limbic-related afferents, particularly the hypothalamus, and mainly distributes to "affective" structures of the forebrain including the bed nucleus of stria terminalis, nucleus accumbens and the amygdala. Accordingly, PV serves a critical role in "motivated behaviors" such as arousal, feeding/consummatory behavior and drug addiction. The rostral ILt receives both limbic and sensorimotor-related input and distributes widely over limbic and motor regions of the frontal cortex-and throughout the dorsal striatum. The intralaminar thalamus is critical for maintaining consciousness and directly participates in various sensorimotor functions (visuospatial or reaction time tasks) and cognitive tasks involving striatal-cortical interactions. As discussed herein, while each of the midline and intralaminar nuclei are anatomically and functionally distinct, they collectively serve a vital role in several affective, cognitive and executive behaviors - as major components of a brainstem-diencephalic-thalamocortical circuitry.
Keywords: affect; arousal; cognition; hippocampus; limbic thalamus; medial prefrontal cortex; striatum.
Copyright © 2022 Vertes, Linley and Rojas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
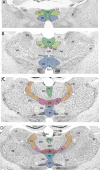
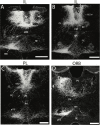
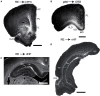
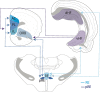
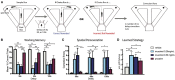
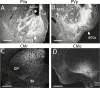
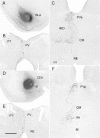
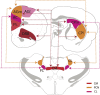

References
-
- Arias-Garcia M. A., Tapia D., Laville J. A., Calderon V. M., Ramiro-Cortes Y., Bargas J., et al. . (2017). Functional comparison of corticostriatal and thalamostriatal postsynaptic responses in striatal neurons of the mouse. Brain Struct. Funct. 223, 1229–1253. 10.1007/s00429-017-1536-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

