Pediatric Ventilation Liberation: A Survey of International Practice Among 555 Pediatric Intensivists
- PMID: 36082374
- PMCID: PMC9444408
- DOI: 10.1097/CCE.0000000000000756
Pediatric Ventilation Liberation: A Survey of International Practice Among 555 Pediatric Intensivists
Abstract
Pediatric ventilation liberation has limited evidence, likely resulting in wide practice variation. To inform future work, practice patterns must first be described.
Objectives: Describe international pediatric ventilation liberation practices and regional practice variation.
Design setting and participants: International cross-sectional electronic survey. Nontrainee pediatric medical and cardiac critical care physicians.
Main outcomes and measures: Practices focusing on spontaneous breathing trial (SBT) eligibility, SBT practice, non-SBT extubation readiness bundle elements, and post-extubation respiratory support.
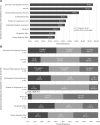
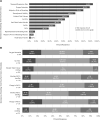
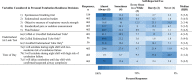
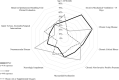
Results: Five-hundred fifty-five responses representing 47 countries were analyzed. Most respondents reported weaning followed by an SBT (86.4%). The top SBT eligibility variables reported were positive end-expiratory pressure (95%), Fio2 (93.4%), and peak inspiratory pressure (73.9%). Most reported use of standardized pressure support regardless of endotracheal tube size (40.4%) with +10 cm H2O predominating (38.6%). SBT durations included less than or equal to 30 minutes (34.8%), 31 minutes to 1 hour (39.3%), and greater than 1 hours (26%). In assigning an SBT result, top variables were respiratory rate (94%), oxygen saturation (89.3%), and subjective work of breathing (79.8%). Most reported frequent consideration of endotracheal secretion burden (81.3%), standardized pain/sedation measurement (72.8%), fluid balance (83%), and the endotracheal air leak test as a part of extubation readiness bundles. Most reported using planned high flow nasal cannula in less than or equal to 50% of extubations (83.2%). Top subpopulations supported with planned HFNC were those with chronic lung disease (67.3%), exposed to invasive ventilation greater than 14 days (66.6%), and chronic critical illness (44.9%). Most reported using planned noninvasive ventilation (NIV) following less than or equal to 20% of extubations (79.9%). Top subpopulations supported with planned NIV were those with neuromuscular disease (72.8%), chronic lung disease (66.7%), and chronic NIV use for any reason (61.6%). Regional variation was high for most practices studied.
Conclusion and relevance: International pediatric ventilation liberation practices are heterogeneous. Future study is needed to address key evidence gaps. Many practice differences were associated with respondent region, which must be considered in international study design.
Keywords: clinical pathway; extubation; mechanical ventilation; pediatric intensive care unit; pediatrics; respiratory therapy.
Copyright © 2022 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Figures
References
-
- Khemani RG, Markovitz BP, Curely MAQ. Epidemiologic factors of mechanically ventilated PICU patients in the United States. Pediatr Crit Care Med 2007; 8:A39
-
- Rivera R, Tibballs J: Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Med 1992; 20:193–199 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

