Risk stratification and early detection biomarkers for precision HCC screening
- PMID: 36082510
- PMCID: PMC9995677
- DOI: 10.1002/hep.32779
Risk stratification and early detection biomarkers for precision HCC screening
Abstract
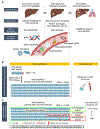
Hepatocellular carcinoma (HCC) mortality remains high primarily due to late diagnosis as a consequence of failed early detection. Professional societies recommend semi-annual HCC screening in at-risk patients with chronic liver disease to increase the likelihood of curative treatment receipt and improve survival. However, recent dynamic shift of HCC etiologies from viral to metabolic liver diseases has significantly increased the potential target population for the screening, whereas annual incidence rate has become substantially lower. Thus, with the contemporary HCC etiologies, the traditional screening approach might not be practical and cost-effective. HCC screening consists of (i) definition of rational at-risk population, and subsequent (ii) repeated application of early detection tests to the population at regular intervals. The suboptimal performance of the currently available HCC screening tests highlights an urgent need for new modalities and strategies to improve early HCC detection. In this review, we overview recent developments of clinical, molecular, and imaging-based tools to address the current challenge, and discuss conceptual framework and approaches of their clinical translation and implementation. These encouraging progresses are expected to transform the current "one-size-fits-all" HCC screening into individualized precision approaches to early HCC detection and ultimately improve the poor HCC prognosis in the foreseeable future.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflict of interest:
J.Y. serves as consultant for Exact Sciences, Exelixis, and Eisai. Y.H. serves as an advisory board member for Helio Genomics and Espervita Therapeutics, and share holder for Alentis Therapeutics and Espervita Therapeutics.
Figures


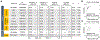
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Lee YT, Wang JJ, Luu M, Tseng HR, Rich NE, Lu SC, Nissen NN, et al. State-Level HCC Incidence and Association With Obesity and Physical Activity in the United States. Hepatology 2021;74:1384–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA226052/CA/NCI NIH HHS/United States
- CA255621/NH/NIH HHS/United States
- DK099558/NH/NIH HHS/United States
- R01 CA222900/CA/NCI NIH HHS/United States
- CA233794/NH/NIH HHS/United States
- U01 CA230694/CA/NCI NIH HHS/United States
- CA230694/NH/NIH HHS/United States
- CA191051/NH/NIH HHS/United States
- R01 CA233794/CA/NCI NIH HHS/United States
- R01 DK099558/DK/NIDDK NIH HHS/United States
- R01 CA255621/CA/NCI NIH HHS/United States
- CA222900/NH/NIH HHS/United States
- 671231/ERC_/European Research Council/International
- 101021417/ERC_/European Research Council/International
LinkOut - more resources
Full Text Sources
Medical

