Resveratrol inhibits development of colorectal adenoma via suppression of LEF1; comprehensive analysis with connectivity map
- PMID: 36082704
- PMCID: PMC9746064
- DOI: 10.1111/cas.15576
Resveratrol inhibits development of colorectal adenoma via suppression of LEF1; comprehensive analysis with connectivity map
Abstract
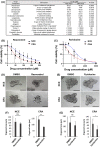
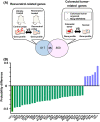
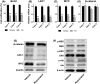
Although many chemopreventive studies on colorectal tumors have been reported, no effective and safe preventive agent is currently available. We searched for candidate preventive compounds against colorectal tumor comprehensively from United States Food and Drug Administration (FDA)-approved compounds by using connectivity map (CMAP) analysis coupled with in vitro screening with colorectal adenoma (CRA) patient-derived organoids (PDOs). We generated CRA-specific gene signatures based on the DNA microarray analysis of CRA and normal epithelial specimens, applied them to CMAP analysis with 1309 FDA-approved compounds, and identified 121 candidate compounds that should cancel the gene signatures. We narrowed them down to 15 compounds, and evaluated their inhibitory effects on the growth of CRA-PDOs in vitro. We finally identified resveratrol, one of the polyphenolic phytochemicals, as a compound showing the strongest inhibitory effect on the growth of CRA-PDOs compared with normal epithelial PDOs. When resveratrol was administered to ApcMin/+ mice at 15 or 30 mg/kg, the number of polyps (adenomas) was significantly reduced in both groups compared with control mice. Similarly, the number of polyps (adenomas) was significantly reduced in azoxymethane-injected rats treated with 10 or 100 mg/resveratrol compared with control rats. Microarray analysis of adenomas from resveratrol-treated rats revealed the highest change (downregulation) in expression of LEF1, a key molecule in the Wnt signaling pathway. Treatment with resveratrol significantly downregulated the Wnt-target gene (MYC) in CRA-PDOs. Our data demonstrated that resveratrol can be the most effective compound for chemoprevention of colorectal tumors, the efficacy of which is mediated through suppression of LEF1 expression in the Wnt signaling pathway.
Keywords: LEF1; chemoprevention; colorectal adenoma; connectivity map; resveratrol.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Lansoprazole inhibits the development of sessile serrated lesions by inducing G1 arrest via Skp2/p27 signaling pathway.J Gastroenterol. 2024 Jan;59(1):11-23. doi: 10.1007/s00535-023-02052-0. Epub 2023 Nov 22. J Gastroenterol. 2024. PMID: 37989907
-
5-Aminosalicylic acid inhibits stem cell function in human adenoma-derived cells: implications for chemoprophylaxis in colorectal tumorigenesis.Br J Cancer. 2021 Jun;124(12):1959-1969. doi: 10.1038/s41416-021-01354-5. Epub 2021 Mar 30. Br J Cancer. 2021. PMID: 33785874 Free PMC article.
-
Interrogating colorectal cancer metastasis to liver: a search for clinically viable compounds and mechanistic insights in colorectal cancer Patient Derived Organoids.J Exp Clin Cancer Res. 2023 Jul 17;42(1):170. doi: 10.1186/s13046-023-02754-6. J Exp Clin Cancer Res. 2023. PMID: 37460938 Free PMC article.
-
Aspirin and other nonsteroidal anti-inflammatory agents in the prevention of colorectal cancer.Important Adv Oncol. 1996:123-37. Important Adv Oncol. 1996. PMID: 8791132 Review.
-
[In situ analyses of molecular mechanisms of colorectal carcinogenesis].Pathologe. 2013 Nov;34 Suppl 2:269-73. doi: 10.1007/s00292-013-1821-y. Pathologe. 2013. PMID: 24196627 Review. German.
Cited by
-
Tumor organoids in cancer medicine: from model systems to natural compound screening.Pharm Biol. 2025 Dec;63(1):89-109. doi: 10.1080/13880209.2025.2458149. Epub 2025 Feb 1. Pharm Biol. 2025. PMID: 39893515 Free PMC article. Review.
-
Lansoprazole inhibits the development of sessile serrated lesions by inducing G1 arrest via Skp2/p27 signaling pathway.J Gastroenterol. 2024 Jan;59(1):11-23. doi: 10.1007/s00535-023-02052-0. Epub 2023 Nov 22. J Gastroenterol. 2024. PMID: 37989907
-
Anti-Inflammatory Effects of Dietary Polyphenols through Inhibitory Activity against Metalloproteinases.Molecules. 2023 Jul 15;28(14):5426. doi: 10.3390/molecules28145426. Molecules. 2023. PMID: 37513300 Free PMC article. Review.
-
LEF1 influences diabetic retinopathy and retinal pigment epithelial cell ferroptosis via the miR-495-3p/GRP78 axis through lnc-MGC.World J Diabetes. 2025 Mar 15;16(3):92003. doi: 10.4239/wjd.v16.i3.92003. World J Diabetes. 2025. PMID: 40093269 Free PMC article.
-
AMPK/mTOR/ULK1 Pathway Participates in Autophagy Induction by Curcumin in Colorectal Adenoma Mouse Model.Drug Dev Res. 2025 Aug;86(5):e70115. doi: 10.1002/ddr.70115. Drug Dev Res. 2025. PMID: 40635394 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal‐tumor development. N Engl J Med. 1988;319:525‐532. - PubMed
-
- Chan TA. Nonsteroidal anti‐inflammatory drugs, apoptosis, and colon‐cancer chemoprevention. Lancet Oncol. 2002;3:166‐174. - PubMed
-
- Flossmann E, Rothwell PM. Effect of aspirin on long‐term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603‐1613. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

