Use of a novel "Split" ventilation system in bench and porcine modeling of acute respiratory distress syndrome
- PMID: 36082971
- PMCID: PMC9461348
- DOI: 10.14814/phy2.15452
Use of a novel "Split" ventilation system in bench and porcine modeling of acute respiratory distress syndrome
Abstract
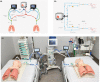
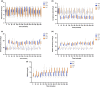
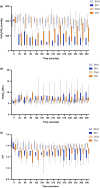
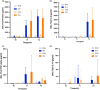
Split ventilation (using a single ventilator to ventilate multiple patients) is technically feasible. However, connecting two patients with acute respiratory distress syndrome (ARDS) and differing lung mechanics to a single ventilator is concerning. This study aimed to: (1) determine functionality of a split ventilation system in benchtop tests, (2) determine whether standard ventilation would be superior to split ventilation in a porcine model of ARDS and (3) assess usability of a split ventilation system with minimal specific training. The functionality of a split ventilation system was assessed using test lungs. The usability of the system was assessed in simulated clinical scenarios. The feasibility of the system to provide modified lung protective ventilation was assessed in a porcine model of ARDS (n = 30). In bench testing a split ventilation system independently ventilated two test lungs under conditions of varying compliance and resistance. In usability tests, a high proportion of naïve operators could assemble and use the system. In the porcine model, modified lung protective ventilation was feasible with split ventilation and produced similar respiratory mechanics, gas exchange and biomarkers of lung injury when compared to standard ventilation. Split ventilation can provide some elements of lung protective ventilation and is feasible in bench testing and an in vivo model of ARDS.
Keywords: acute lung injury; acute respiratory distress syndrome; lung protective ventilation; split ventilation.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The author declare no competing interests.
Figures

References
-
- Angulo, M. , Beltramelli, R. , Amarelle, L. , Alzugaray, P. , Briva, A. , & Santos, C. (2020). Mechanical risks of ventilator sharing in the COVID‐19 era: A simulation‐based study. Archivos de Bronconeumología (English Edition), 56(11), 752–753. - PubMed
-
- Beitler, J. R. , Mittel, A. M. , Kallet, R. , Kacmarek, R. , Hess, D. , Branson, R. , Olson, M. , Garcia, I. , Powell, B. , Wang, D. S. , Hastie, J. , Panzer, O. , Brodie, D. , Hill, L. L. , & Thompson, B. T. (2020). Ventilator sharing during an acute shortage caused by the COVID‐19 pandemic. American Journal of Respiratory and Critical Care Medicine, 202(4), 600–604. - PMC - PubMed
-
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological), 57(1), 289–300.
-
- Bishawi, M. , Kaplan, M. , Chidyagwai, S. , Cappiello, J. , Cherry, A. , MacLeod, D. , Gall, K. , Evans, N. , Kim, M. , Shaha, R. , Whittle, J. , Hollidge, M. , Truskey, G. , & Randles, A. (2020). Rapid ventilator splitting during COVID‐19 pandemic using 3D printed devices and numerical modeling of 200 million patient specific air flow scenarios. Research Square. 10.21203/rs.3.rs-48165/v1 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

