Phomoxanthone A Targets ATP Synthase
- PMID: 36082977
- PMCID: PMC9942271
- DOI: 10.1002/chem.202202397
Phomoxanthone A Targets ATP Synthase
Abstract
Phomoxanthone A is a naturally occurring molecule and a powerful anti-cancer agent, although its mechanism of action is unknown. To facilitate the determination of its biological target(s), we used affinity-based labelling using a phomoxanthone A probe. Labelled proteins were pulled down, subjected to chemoproteomics analysis using LC-MS/MS and ATP synthase was identified as a likely target. Mitochondrial ATP synthase was validated in cultured cells lysates and in live intact cells. Our studies show sixty percent inhibition of ATP synthase by 260 μM phomoxanthone A.
Keywords: anticancer; biconjugation; microscopy; natural product; photoaffinity labelling.
© 2022 Wiley-VCH GmbH.
Figures

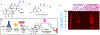
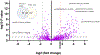




References
-
- Wezeman T, Brase S, Masters K “Xanthone Dimers: a Compound Family which is both Common and Privileged” Nat. Prod. Rep 2015, 32, 6–28. - PubMed
-
- Masters K, Brase S “Xanthones from Fungi, Lichens, and Bacteria: the Natural Products and Their Synthesis” Chem. Rev 2012, 112, 3717–3776. - PubMed
-
- Rönsberg D, Debbab A, Mándi A, Vasylyeva V, Böhler P, Stork B, Engelke L, Hamcher A, Sawadogo R, Diederich M, Wray V, Lin W, Kassack M, Janiak C, Scheu S, Wesselborg S, Kurtán T, Aly A, Proksch P “Prop-Apoptotic and Immunostimulatory Tetrahydroxanthone Dimers for the Endophytic Fungus Phomopsis longicolla” J. Org. Chem 2013, 78, 12409–12425. - PubMed
-
- Böhler P, Stuhldreier F, Anand R, Kondadi A, Schlutermann D, Berleth N, Deitersen J, Wallot-Hieke N, Wu W, Frank M, Niemann H, Wesbuer E, Barbian A, Luyten T, Parys J, Weidtkamp-Peters S, Borchardt A, Reichert A, Pena-Blano A, Garcia-Saez A, Itskanov S, van der Bliek A, Proksch P, Wesselborg S, Stork B, B. “The Mycotoxon Phomoxanthone A Distrubs the Form and Function of the Inner Mitochondrial Membrane” Cell Death & Disease 2018, 9, 286–303. - PMC - PubMed
-
- Wang C, Engelke L, Bickel D, Hamacher A, Frank M, Proksch P, Gohlke H, Kassack M “The Tetrahydroxanthone-Dimer Phomoxanthone A is a Strong Inducer of Apoptosis in Cisplatin-resistant Solid Cancer Cells” Bioorg. Med. Chem 2019, 27, 115044–115056. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

