A Phase I-II multicenter trial with Avelumab plus autologous dendritic cell vaccine in pre-treated mismatch repair-proficient (MSS) metastatic colorectal cancer patients; GEMCAD 1602 study
- PMID: 36083313
- PMCID: PMC10025226
- DOI: 10.1007/s00262-022-03283-5
A Phase I-II multicenter trial with Avelumab plus autologous dendritic cell vaccine in pre-treated mismatch repair-proficient (MSS) metastatic colorectal cancer patients; GEMCAD 1602 study
Abstract
Background: Immune check-point blockade (ICB) has shown clinical benefit in mismatch repair-deficient/microsatellite instability high metastatic colorectal cancer (mCRC) but not in mismatch repair-proficient/microsatellite stable patients. Cancer vaccines with autologous dendritic cells (ADC) could be a complementary therapeutic approach to ICB as this combination has the potential to achieve synergistic effects.
Methods: This was a Phase I/II multicentric study with translational sub-studies, to evaluate the safety, pharmacodynamics and anti-tumor effects of Avelumab plus ADC vaccine in heavily pre-treated MSS mCRC patients. Primary objective was to determine the maximum tolerated dose and the efficacy of the combination. The primary end-point was 40% progression-free survival at 6 months with a 2 Simon Stage.
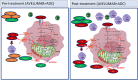
Results: A total of 28 patients were screened and 19 pts were included. Combined therapy was safe and well tolerated. An interim analysis (Simon design first-stage) recommended early termination because only 2/19 (11%) patients were disease free at 6 months. Median PFS was 3.1 months [2.1-5.3 months] and overall survival was 12.2 months [3.2-23.2 months]. Stimulation of immune system was observed in vitro but not clinically. The evaluation of basal RNA-seq noted significant changes between pre and post-therapy liver biopsies related to lipid metabolism and transport, inflammation and oxidative stress pathways.
Conclusions: The combination of Avelumab plus ADC vaccine is safe and well tolerated but exhibited modest clinical activity. Our study describes, for the first-time, a de novo post-therapy metabolic rewiring, that could represent novel immunotherapy-induced tumor vulnerabilities.
Trial registration: ClinicalTrials.gov NCT03152565.
Keywords: Metabolism; Resistance; Vaccines.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C, Beets GL, Snaebjornsson P, Maas M, Mertz M, Veninga V, Bounova G, Broeks A, Beets-Tan RG, de Wijkerslooth TR, van Lent AU, Marsman HA, Nuijten E, Kok NF, Kuiper M, Verbeek WH, Kok M, Van Leerdam ME, Schumacher TN, Voest EE, Haanen JB. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26(4):566–576. doi: 10.1038/s41591-020-0805-8. - DOI - PubMed
-
- Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, García-Alfonso P, Neyns B, Luppi G, Cardin DB, Dragovich T, Shah U, Abdullaev S, Gricar J, Ledeine JM, Overman MJ. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the phase II checkMate 142 study. J Clin Oncol. 2022;40(2):161–170. doi: 10.1200/JCO.21.01015. - DOI - PubMed
-
- Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA., Jr PD-1 blockade in mismatch repair-deficient locally advanced rectal cancer. N Engl J Med. 2022 doi: 10.1056/NEJMoa2201445. - DOI - PMC - PubMed
-
- André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fouchardiere C, Rivera F, Elez E, Bendell J, Le DT, Yoshino T, Van Cutsem E, Yang P, Farooqui MZH, Marinello P, Diaz LA., Jr KEYNOTE-177 investigators. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–2218. doi: 10.1056/NEJMoa2017699. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

