Backbone and side-chain resonance assignments of the NISTmAb-scFv and antigen-binding study
- PMID: 36083574
- PMCID: PMC9510101
- DOI: 10.1007/s12104-022-10109-z
Backbone and side-chain resonance assignments of the NISTmAb-scFv and antigen-binding study
Abstract
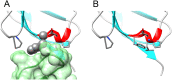
Monoclonal antibodies (mAbs) therapeutics are the largest and fastest growing class of biologic drugs, amongst which, the vast majority are immunoglobulin G1 (IgG1). Their antigen binding abilities are used for the treatment of immunologic diseases, cancer therapy, reversal of drug effects, and targeting viruses and bacteria. The high importance of therapeutic mAbs and their derivatives has called for the generation of well-characterized standards for method development and calibration. One such standard, the NISTmAb RM 8621 based on the antibody motavizumab, has been developed by the National Institute of Standards and Technologies (NIST) in the US. Here, we present the resonance assignment of the single chain variable fragment, NISTmAb-scFv, that was engineered by linking the variable domains of the heavy and light chains of the NISTmAb. Also, addition of a peptide, corresponding to the target antigen of motavizumab, to samples of NISTmAb-scFv has induced chemical shift perturbations on residues lining the antigen binding interface thereby indicating proper folding of the NISTmAb-scFv.
Keywords: Monoclonal antibody; Motavizumab; NISTmAb; NMR spectroscopy; Respiratory syncytial virus; Single-chain variable fragment.
© 2022. Crown.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

