Redox-controlled reorganization and flavin strain within the ribonucleotide reductase R2b-NrdI complex monitored by serial femtosecond crystallography
- PMID: 36083619
- PMCID: PMC9462851
- DOI: 10.7554/eLife.79226
Redox-controlled reorganization and flavin strain within the ribonucleotide reductase R2b-NrdI complex monitored by serial femtosecond crystallography
Abstract
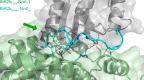
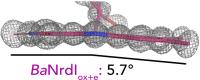
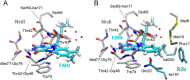
Redox reactions are central to biochemistry and are both controlled by and induce protein structural changes. Here, we describe structural rearrangements and crosstalk within the Bacillus cereus ribonucleotide reductase R2b-NrdI complex, a di-metal carboxylate-flavoprotein system, as part of the mechanism generating the essential catalytic free radical of the enzyme. Femtosecond crystallography at an X-ray free electron laser was utilized to obtain structures at room temperature in defined redox states without suffering photoreduction. Together with density functional theory calculations, we show that the flavin is under steric strain in the R2b-NrdI protein complex, likely tuning its redox properties to promote superoxide generation. Moreover, a binding site in close vicinity to the expected flavin O2 interaction site is observed to be controlled by the redox state of the flavin and linked to the channel proposed to funnel the produced superoxide species from NrdI to the di-manganese site in protein R2b. These specific features are coupled to further structural changes around the R2b-NrdI interaction surface. The mechanistic implications for the control of reactive oxygen species and radical generation in protein R2b are discussed.
Keywords: bacillus cereus; biochemistry; chemical biology; flavoprotein; metalloprotein; molecular biophysics; oxidoreductase; oxygen activation; ribonucleotide reductase; serial femtosecond crystallography; structural biology.
© 2022, John et al.
Conflict of interest statement
JJ, OA, VS, PS, IK, AB, PS, MD, CP, SG, KS, PA, AB, AO, MC, SO, KT, FF, AB, AB, NS, VY, JY, VK, JK, HL, MH No competing interests declared
Figures









Similar articles
-
Crystal structure of Bacillus cereus class Ib ribonucleotide reductase di-iron NrdF in complex with NrdI.ACS Chem Biol. 2014 Feb 21;9(2):526-37. doi: 10.1021/cb400757h. Epub 2013 Dec 11. ACS Chem Biol. 2014. PMID: 24295378
-
High-resolution crystal structures of the flavoprotein NrdI in oxidized and reduced states--an unusual flavodoxin. Structural biology.FEBS J. 2010 Oct;277(20):4265-77. doi: 10.1111/j.1742-4658.2010.07815.x. Epub 2010 Sep 10. FEBS J. 2010. PMID: 20831589
-
Redox-induced structural changes in the di-iron and di-manganese forms of Bacillus anthracis ribonucleotide reductase subunit NrdF suggest a mechanism for gating of radical access.J Biol Inorg Chem. 2019 Sep;24(6):849-861. doi: 10.1007/s00775-019-01703-z. Epub 2019 Aug 13. J Biol Inorg Chem. 2019. PMID: 31410573 Free PMC article.
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Metal use in ribonucleotide reductase R2, di-iron, di-manganese and heterodinuclear--an intricate bioinorganic workaround to use different metals for the same reaction.Metallomics. 2011 Feb;3(2):110-20. doi: 10.1039/c0mt00095g. Epub 2011 Jan 25. Metallomics. 2011. PMID: 21267492 Review.
Cited by
-
Characterization of a second class Ie ribonucleotide reductase.Commun Biol. 2025 Feb 22;8(1):281. doi: 10.1038/s42003-025-07565-3. Commun Biol. 2025. PMID: 39987380 Free PMC article.
-
Structure of a ribonucleotide reductase R2 protein radical.Science. 2023 Oct 6;382(6666):109-113. doi: 10.1126/science.adh8160. Epub 2023 Oct 5. Science. 2023. PMID: 37797025 Free PMC article.
-
Room temperature crystallography and X-ray spectroscopy of metalloenzymes.Methods Enzymol. 2023;688:307-348. doi: 10.1016/bs.mie.2023.07.009. Epub 2023 Aug 16. Methods Enzymol. 2023. PMID: 37748830 Free PMC article.
-
Why is manganese so valuable to bacterial pathogens?Front Cell Infect Microbiol. 2023 Feb 3;13:943390. doi: 10.3389/fcimb.2023.943390. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36816586 Free PMC article. Review.
-
Manganese uptake by MtsABC contributes to the pathogenesis of human pathogen group A streptococcus by resisting host nutritional immune defenses.Infect Immun. 2024 Jul 11;92(7):e0007724. doi: 10.1128/iai.00077-24. Epub 2024 Jun 13. Infect Immun. 2024. PMID: 38869295 Free PMC article.
References
-
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallographica. Section D, Biological Crystallography. 2012;68:352–367. doi: 10.1107/S0907444912001308. - DOI - PMC - PubMed
-
- Balasubramani SG, Chen GP, Coriani S, Diedenhofen M, Frank MS, Franzke YJ, Furche F, Grotjahn R, Harding ME, Hättig C, Hellweg A, Helmich-Paris B, Holzer C, Huniar U, Kaupp M, Marefat Khah A, Karbalaei Khani S, Müller T, Mack F, Nguyen BD, Parker SM, Perlt E, Rappoport D, Reiter K, Roy S, Rückert M, Schmitz G, Sierka M, Tapavicza E, Tew DP, van Wüllen C, Voora VK, Weigend F, Wodyński A, Yu JM. TURBOMOLE: modular program suite for ab initio quantum-chemical and condensed-matter simulations. J Chem Phys. 2020;152:184107. doi: 10.1063/5.0004635. - DOI - PMC - PubMed
-
- Becke AD. Density‐functional thermochemistry. III. the role of exact exchange. J Chem Phys. 1993;98:5648–5652. doi: 10.1063/1.464913. - DOI
-
- Blaesi EJ, Palowitch GM, Hu K, Kim AJ, Rose HR, Alapati R, Lougee MG, Kim HJ, Taguchi AT, Tan KO, Laremore TN, Griffin RG, Krebs C, Matthews ML, Silakov A, Bollinger JM, Allen BD, Boal AK. Metal-free class ie ribonucleotide reductase from pathogens initiates catalysis with a tyrosine-derived dihydroxyphenylalanine radical. PNAS. 2018;115:10022–10027. doi: 10.1073/pnas.1811993115. - DOI - PMC - PubMed

