Efficacy of rituximab in anti-myelin-associated glycoprotein demyelinating polyneuropathy: Clinical, hematological and neurophysiological correlations during 2 years of follow-up
- PMID: 36083713
- PMCID: PMC9825860
- DOI: 10.1111/ene.15553
Efficacy of rituximab in anti-myelin-associated glycoprotein demyelinating polyneuropathy: Clinical, hematological and neurophysiological correlations during 2 years of follow-up
Abstract
Background and purpose: We evaluated the clinical and neurophysiological efficacy of rituximab (RTX) in a neurophysiologically homogeneous group of patients with monoclonal gammopathy and immunoglobulin M (IgM) anti-myelin-associated glycoprotein antibody (anti-MAG) demyelinating polyneuropathy.
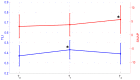
Methods: Twenty three anti-MAG-positive polyneuropathic patients were prospectively evaluated before and for 2 years after treatment with RTX 375 mg/m2 . The Inflammatory Neuropathy Cause and Treatment (INCAT) disability scale (INCAT-ds), modified INCAT sensory score (mISS), Medical Research Council sum score, Patients' Global Impression of Change scale were used, IgM levels were assessed and extensive electrophysiological examinations were performed before (T0) and 1 year (T1) and 2 years (T2) after RTX treatment.
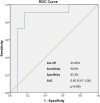
Results: At T1 and T2 there was a significant reduction from T0 both in mISS and in INCAT-ds, with a p value < 0.001 in the inferential Friedman's test overall analysis. Ulnar nerve Terminal Latency Index and distal motor latency significantly changed from T0 to T1 and in the overall analysis (p = 0.001 and p = 0.002), and ulnar nerve sensory nerve action potential (SNAP) amplitude was significantly increased at T2 from T1, with a p value < 0.001 in the overall analysis. Analysis of the receiver-operating characteristic curves showed that a 41.8% increase in SNAP amplitude in the ulnar nerve at T2 from T0 was a fair predictor of a mISS reduction of ≥2 points (area under the curve 0.85; p = 0.005; sensitivity: 90.9%, specificity: 83.3%).
Conclusions: This study suggests that RTX is effective in patients with clinically active demyelinating anti-MAG neuropathy over 2 years of follow-up, and that some neurophysiological variables might be useful for monitoring this efficacy.
Keywords: clinical neurophysiology; haematological disorders; immunomodulatory therapy; polyneuropathy.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures

References
-
- Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long‐term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–9. - PubMed
-
- Yeung KB, Thomas PK, King RH, et al. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. J Neurol. 1991;238(7):383‐391. - PubMed
-
- Capasso M, Torrieri F, Di Muzio A, De Angelis MV, Lugaresi A, Uncini A. Can electrophysiology differentiate polyneuropathy with anti‐MAG/SGPG antibodies from chronic inflammatory demyelinating polyneuropathy? Clin Neurophysiol. 2002;113(3):346‐353. - PubMed
-
- Magy L, Chassande B, Maisonobe T, Bouche P, Vallat JM, Léger JM. Polyneuropathy associated with IgG/IgA monoclonal gammopathy: a clinical and electrophysiological study of 15 cases. Eur J Neurol. 2003;10(6):677‐685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

