Colorectal cancers with a residual adenoma component: Clinicopathologic features and KRAS mutation
- PMID: 36083889
- PMCID: PMC9462729
- DOI: 10.1371/journal.pone.0273723
Colorectal cancers with a residual adenoma component: Clinicopathologic features and KRAS mutation
Erratum in
-
Correction: Colorectal cancers with a residual adenoma component: Clinicopathologic features and KRAS mutation.PLoS One. 2025 Oct 17;20(10):e0334953. doi: 10.1371/journal.pone.0334953. eCollection 2025. PLoS One. 2025. PMID: 41105578 Free PMC article.
Abstract
Background/aim: Colorectal cancer is well known for its "adenoma-carcinoma" sequential carcinogenesis. Some colorectal cancers demonstrate a residual adenoma component during progression from adenoma to invasive carcinoma. However, the clinicopathological significance of residual adenoma component remains unclear. In this study, we aimed to investigate the clinicopathologic and molecular characteristics including the KRAS mutation in colorectal cancers containing a residual adenoma component.
Materials and methods: In this study, 498 surgically resected colorectal cancer patients were enrolled. Their detailed clinicopathologic features and results of molecular study including KRAS mutation test and microsatellite instability were analyzed.
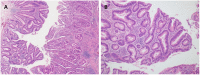
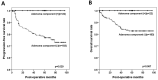
Results: A residual adenoma component was identified in 42 (8.4%) patients with colorectal cancer. The presence of a residual adenoma component was associated with a high frequency of the KRAS mutation (65%, p = 0.031) as well as indolent clinicopathological features, including polypoid gross type (p < 0.001), well-differentiated histology (p < 0.001), low pT (p < 0.001) and pN stage (p = 0.003), absence of vascular invasion (p = 0.005), and a better progression-free prognosis (p = 0.029). The cases with an adenoma component had a 35.7% discordance rate on the KRAS mutation tests in their adenoma and carcinoma regions.
Conclusion: In conclusion, colorectal cancer with a residual adenoma component showed indolent clinicopathologic features and frequent KRAS mutations. Due to the discordance in the incidence of the KRAS mutation between the adenoma and carcinoma components, the adenoma component should be documented in the pathology report, and care should be taken not to include the adenoma component when collecting samples for molecular testing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Smit WL, Spaan CN, Johannes de Boer R, Ramesh P, Martins Garcia T, Meijer BJ, et al. Driver mutations of the adenoma-carcinoma sequence govern the intestinal epithelial global translational capacity. Proceedings of the National Academy of Sciences. 2020;117(41):25560–70. doi: 10.1073/pnas.1912772117 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

