Extracellular Matrix Modulates Outgrowth Dynamics in Ovarian Cancer
- PMID: 36084257
- PMCID: PMC9772079
- DOI: 10.1002/adbi.202200197
Extracellular Matrix Modulates Outgrowth Dynamics in Ovarian Cancer
Abstract
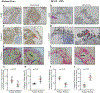
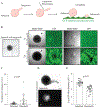
Ovarian carcinoma (OC) forms outgrowths that extend from the outer surface of an afflicted organ into the peritoneum. OC outgrowth formation is poorly understood due to the limited availability of cell culture models examining the behavior of cells that form outgrowths. Prompted by immunochemical evaluation of extracellular matrix (ECM) components in human tissues, laminin and collagen-rich ECM-reconstituted cell culture models amenable to studies of cell clusters that can form outgrowths are developed. It is demonstrated that ECM promotes outgrowth formation in fallopian tube non-ciliated epithelial cells (FNE) expressing mutant p53 and various OC cell lines. Outgrowths are initiated by cells that underwent outward translocation and retained the ability to intercalate into mesothelial cell monolayers. Electron microscopy, optical coherence tomography, and small amplitude oscillatory shear experiments reveal that increased ECM levels led to increased fibrous network thickness and high shear elasticity of the microenvironment. These physical characteristics are associated with outgrowth suppression. The low ECM microenvironment mimicks the viscoelasticity of malignant peritoneal fluid (ascites) and supports cell proliferation, cell translocation, and outgrowth formation. These results highlight the importance of the ECM microenvironment in modulating OC growth and can provide additional insights into the mode of dissemination of primary and recurrent ovarian tumors.
Keywords: ascites; collagen; extracellular matrix; laminin γ1; outgrowths; ovarian cancer; tumor microenvironment.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Figures





References
-
- Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol [Internet] 2010. [cited 2022 Apr 16];34(3):433–43. Available from: https://pubmed.ncbi.nlm.nih.gov/20154587/ - PMC - PubMed
-
- Auersperg N Ovarian surface epithelium as a source of ovarian cancers: unwarranted speculation or evidence-based hypothesis? Gynecol Oncol [Internet] 2013. [cited 2022 Apr 16];130(1):246–51. Available from: https://pubmed.ncbi.nlm.nih.gov/23558054/ - PubMed
-
- Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, et al. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol [Internet] 2008. [cited 2022 Apr 16];109(2):168–73. Available from: https://pubmed.ncbi.nlm.nih.gov/18342932/ - PMC - PubMed
-
- Meserve EEK, Brouwer J, Crum CP. Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol [Internet] 2017. [cited 2022 Apr 16];30(5):710–21. Available from: https://pubmed.ncbi.nlm.nih.gov/28106106/ - PubMed
-
- Karst AM, Drapkin R. Ovarian cancer pathogenesis: a model in evolution. J Oncol [Internet] 2010. [cited 2022 Apr 16];2010:1–13. Available from: https://pubmed.ncbi.nlm.nih.gov/19746182/ - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

