Trafficking between clonally related peripheral T-helper cells and tissue-resident T-helper cells in chronic GVHD
- PMID: 36084473
- PMCID: PMC9935547
- DOI: 10.1182/blood.2022016581
Trafficking between clonally related peripheral T-helper cells and tissue-resident T-helper cells in chronic GVHD
Abstract
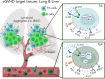
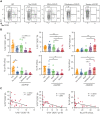
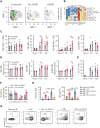
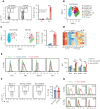
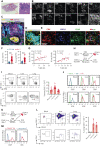
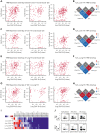
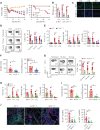
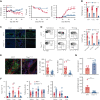
Chronic graft-versus-host disease (cGVHD) is an autoimmune-like syndrome. CXCR5-PD-1hi peripheral T-helper (Tph) cells have an important pathogenic role in autoimmune diseases, but the role of Tph cells in cGVHD remains unknown. We show that in patients with cGVHD, expansion of Tph cells among blood CD4+ T cells was associated with cGVHD severity. These cells augmented memory B-cell differentiation and production of immunoglobulin G via interleukin 21 (IL-21). Tph cell expansion was also observed in a murine model of cGVHD. This Tph cell expansion in the blood is associated with the expansion of pathogenic tissue-resident T-helper (Trh) cells that form lymphoid aggregates surrounded by collagen in graft-versus-host disease (GVHD) target tissues. Adoptive transfer experiments showed that Trh cells from GVHD target tissues give rise to Tph cells in the blood, and conversely, Tph cells from the blood give rise to Trh cells in GVHD target tissues. Tph cells in the blood and Trh cells in GVHD target tissues had highly overlapping T-cell receptor α and β repertoires. Deficiency of IL-21R, B-cell lymphoma 6 (BCL6), or T-bet in donor T cells markedly reduced the proportions of Tph cells in the blood and Trh cells in GVHD target tissues and reduced T-B interaction in the lymphoid aggregates. These results indicate that clonally related pathogenic Tph cells and Trh cells traffic between the blood and cGVHD target tissues, and that IL-21R-BCL6 signaling and T-bet are required for the development and expansion of Tph and Trh cells in the pathogenesis of cGVHD.
© 2022 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Comment in
-
Chronic GVHD on the move.Blood. 2022 Dec 22;140(25):2660-2661. doi: 10.1182/blood.2022018321. Blood. 2022. PMID: 36548018 Free PMC article. No abstract available.
References
-
- Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–2579. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

