Deep mutational scanning identifies SARS-CoV-2 Nucleocapsid escape mutations of currently available rapid antigen tests
- PMID: 36084631
- PMCID: PMC9420710
- DOI: 10.1016/j.cell.2022.08.010
Deep mutational scanning identifies SARS-CoV-2 Nucleocapsid escape mutations of currently available rapid antigen tests
Abstract
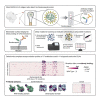
The effects of mutations in continuously emerging variants of SARS-CoV-2 are a major concern for the performance of rapid antigen tests. To evaluate the impact of mutations on 17 antibodies used in 11 commercially available antigen tests with emergency use authorization, we measured antibody binding for all possible Nucleocapsid point mutations using a mammalian surface-display platform and deep mutational scanning. The results provide a complete map of the antibodies' epitopes and their susceptibility to mutational escape. Our data predict no vulnerabilities for detection of mutations found in variants of concern. We confirm this using the commercial tests and sequence-confirmed COVID-19 patient samples. The antibody escape mutational profiles generated here serve as a valuable resource for predicting the performance of rapid antigen tests against past, current, as well as any possible future variants of SARS-CoV-2, establishing the direct clinical and public health utility of our system.
Keywords: SARS-CoV-2 Nucleocapsid protein; antibodies; deep mutational scanning; epitope mapping; mammalian surface-display; rapid diagnostic tests; variants.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures











References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

