Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury
- PMID: 36085050
- PMCID: PMC9462075
- DOI: 10.1186/s13073-022-01108-9
Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury
Abstract
Background: Acute kidney injury (AKI) occurs frequently in critically ill patients and is associated with adverse outcomes. Cellular mechanisms underlying AKI and kidney cell responses to injury remain incompletely understood.
Methods: We performed single-nuclei transcriptomics, bulk transcriptomics, molecular imaging studies, and conventional histology on kidney tissues from 8 individuals with severe AKI (stage 2 or 3 according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria). Specimens were obtained within 1-2 h after individuals had succumbed to critical illness associated with respiratory infections, with 4 of 8 individuals diagnosed with COVID-19. Control kidney tissues were obtained post-mortem or after nephrectomy from individuals without AKI.
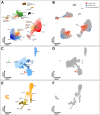
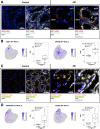
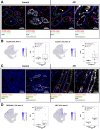
Results: High-depth single cell-resolved gene expression data of human kidneys affected by AKI revealed enrichment of novel injury-associated cell states within the major cell types of the tubular epithelium, in particular in proximal tubules, thick ascending limbs, and distal convoluted tubules. Four distinct, hierarchically interconnected injured cell states were distinguishable and characterized by transcriptome patterns associated with oxidative stress, hypoxia, interferon response, and epithelial-to-mesenchymal transition, respectively. Transcriptome differences between individuals with AKI were driven primarily by the cell type-specific abundance of these four injury subtypes rather than by private molecular responses. AKI-associated changes in gene expression between individuals with and without COVID-19 were similar.
Conclusions: The study provides an extensive resource of the cell type-specific transcriptomic responses associated with critical illness-associated AKI in humans, highlighting recurrent disease-associated signatures and inter-individual heterogeneity. Personalized molecular disease assessment in human AKI may foster the development of tailored therapies.
Keywords: Acute kidney injury; Critical illness; Single-cell sequencing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

