An ensemble recruited by α2a-adrenergic receptors is engaged in a stressor-specific manner in mice
- PMID: 36085168
- PMCID: PMC10267140
- DOI: 10.1038/s41386-022-01442-x
An ensemble recruited by α2a-adrenergic receptors is engaged in a stressor-specific manner in mice
Abstract
α2a-adrenergic receptor (α2a-AR) agonists are candidate substance use disorder therapeutics due to their ability to recruit noradrenergic autoreceptors to dampen stress system engagement. However, we recently found that postsynaptic α2a-ARs are required for stress-induced reinstatement of cocaine-conditioned behavior. Understanding the ensembles recruited by these postsynaptic receptors (heteroceptors) is necessary to understand noradrenergic circuit control. We utilized a variety of approaches in FosTRAP (Targeted Recombination in Active Populations) mice to define an ensemble of cells activated by the α2a-AR partial agonist guanfacine ("Guansembles") in the bed nucleus of the stria terminalis (BST/BNST), a region key to stress-induced reinstatement of drug seeking. We define BNST "Guansembles" and show they differ from restraint stress-activated cells. Guanfacine produced inhibition of cAMP-dependent signaling in Guansembles, while chronic restraint stress increased cAMP-dependent signaling. Guanfacine both excited and inhibited aspects of Guansemble neuronal activity. Further, while some stressors produced overall reductions in Guansemble activity, active coping events during restraint stress and exposure to unexpected shocks were both associated with Guansemble recruitment. Using viral tracing, we define a BNST Guansemble afferent network that includes regions involved in the interplay of stress and homeostatic functions. Finally, we show that activation of Guansembles produces alterations in behavior on the elevated plus maze consistent with task-specific anxiety-like behavior. Overall, we define a population of BNST neurons recruited by α2a-AR signaling that opposes the behavioral action of canonical autoreceptor α2a-AR populations and which are differentially recruited by distinct stressors. Moreover, we demonstrate stressor-specific physiological responses in a specific neuronal population.
© 2022. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures
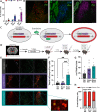
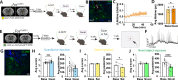



References
-
- Gowing L, Farrell M, Ali R & White JM. Alpha2 adrenergic agonists for the management of opioid withdrawal. in Cochrane Database of Systematic Reviews (ed. The Cochrane Collaboration) CD002024.pub2 (John Wiley & Sons, Ltd, 2004). 10.1002/14651858.CD002024.pub2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

