Higher sensitivity monitoring of reactions to COVID-19 vaccination using smartwatches
- PMID: 36085312
- PMCID: PMC9461410
- DOI: 10.1038/s41746-022-00683-w
Higher sensitivity monitoring of reactions to COVID-19 vaccination using smartwatches
Abstract
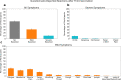
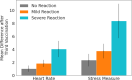
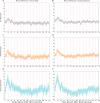
More than 12 billion COVID-19 vaccination shots have been administered as of August 2022, but information from active surveillance about vaccine safety is limited. Surveillance is generally based on self-reporting, making the monitoring process subjective. We study participants in Israel who received their second or third Pfizer BioNTech COVID-19 vaccination. All participants wore a Garmin Vivosmart 4 smartwatch and completed a daily questionnaire via smartphone. We compare post-vaccination smartwatch heart rate data and a Garmin-computed stress measure based on heart rate variability with data from the patient questionnaires. Using a mixed effects panel regression to remove participant-level fixed and random effects, we identify considerable changes in smartwatch measures in the 72 h post-vaccination even among participants who reported no side effects in the questionnaire. Wearable devices were more sensitive than questionnaires in determining when participants returned to baseline levels. We conclude that wearable devices can detect physiological responses following vaccination that may not be captured by patient self-reporting. More broadly, the ubiquity of smartwatches provides an opportunity to gather improved data on patient health, including active surveillance of vaccine safety.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Prediction and detection of side effects severity following COVID-19 and influenza vaccinations: utilizing smartwatches and smartphones.Sci Rep. 2024 Mar 12;14(1):6012. doi: 10.1038/s41598-024-56561-w. Sci Rep. 2024. PMID: 38472345 Free PMC article.
-
Comparison of physiological and clinical reactions to COVID-19 and influenza vaccination.Commun Med (Lond). 2024 Aug 24;4(1):169. doi: 10.1038/s43856-024-00588-7. Commun Med (Lond). 2024. PMID: 39181950 Free PMC article.
-
Continuous Monitoring of Heart Rate Variability in Free-Living Conditions Using Wearable Sensors: Exploratory Observational Study.JMIR Form Res. 2024 Aug 7;8:e53977. doi: 10.2196/53977. JMIR Form Res. 2024. PMID: 39110968 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Allergic reactions to Japanese encephalitis vaccine.Immunol Allergy Clin North Am. 2003 Nov;23(4):665-97. doi: 10.1016/s0889-8561(03)00102-4. Immunol Allergy Clin North Am. 2003. PMID: 14753386 Review.
Cited by
-
Prediction and detection of side effects severity following COVID-19 and influenza vaccinations: utilizing smartwatches and smartphones.Sci Rep. 2024 Mar 12;14(1):6012. doi: 10.1038/s41598-024-56561-w. Sci Rep. 2024. PMID: 38472345 Free PMC article.
-
Crime, inequality and public health: a survey of emerging trends in urban data science.Front Big Data. 2023 May 25;6:1124526. doi: 10.3389/fdata.2023.1124526. eCollection 2023. Front Big Data. 2023. PMID: 37303974 Free PMC article. Review.
-
A scoping review of active, participant centred, digital adverse events following immunization (AEFI) surveillance of WHO approved COVID-19 vaccines: A Canadian immunization Research Network study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2293550. doi: 10.1080/21645515.2023.2293550. Epub 2024 Feb 19. Hum Vaccin Immunother. 2024. PMID: 38374618 Free PMC article.
-
Mapping digital health ecosystems in Africa in the context of endemic infectious and non-communicable diseases.NPJ Digit Med. 2023 May 26;6(1):97. doi: 10.1038/s41746-023-00839-2. NPJ Digit Med. 2023. PMID: 37237022 Free PMC article.
-
Smartwatches in healthcare medicine: assistance and monitoring; a scoping review.BMC Med Inform Decis Mak. 2023 Nov 3;23(1):248. doi: 10.1186/s12911-023-02350-w. BMC Med Inform Decis Mak. 2023. PMID: 37924029 Free PMC article.
References
-
- National Academies of Sciences Engineering and Medicine Committee on Framework for Equitable Allocation of Vaccine for the Novel Coronavirus. Framework for Equitable Allocation of COVID-19 Vaccine. (National Academies Press, 2020). - PubMed
LinkOut - more resources
Full Text Sources

