Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis
- PMID: 36085592
- PMCID: PMC9541589
- DOI: 10.1111/asj.13764
Prevention of mastitis in multiparous dairy cows with a previous history of mastitis by oral feeding with probiotic Bacillus subtilis
Abstract
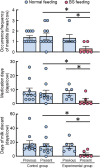
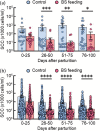
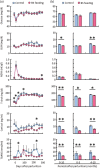
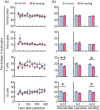
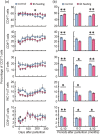
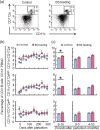
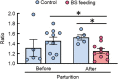
Mastitis is a very common inflammatory disease of the mammary gland of dairy cows, resulting in a reduction of milk production and quality. Probiotics may serve as an alternative to antibiotics to prevent mastitis, and the use of probiotics in this way may lessen the risk of antibiotic resistant bacteria developing. We investigated the effect of oral feeding of probiotic Bacillus subtilis (BS) C-3102 strain on the onset of mastitis in dairy cows with a previous history of mastitis. BS feeding significantly decreased the incidence of mastitis, the average number of medication days and the average number of days when milk was discarded, and maintained the mean SCC in milk at a level substantially lower than the control group. BS feeding was associated with lower levels of cortisol and TBARS and increased the proportion of CD4+ T cells and CD11c+ CD172ahigh dendritic cells in the blood by flow cytometry analysis. Parturition increased the migrating frequency of granulocytes toward a milk chemoattractant cyclophilin A in the control cows, however, this was reduced by BS feeding, possibly indicating a decreased sensitivity of peripheral granulocytes to cyclophilin A. These results reveal that B. subtilis C-3102 has potential as a probiotic and has preventative capacity against mastitis in dairy cows.
Keywords: Bacillus subtilis C-3102 strain; bovine mastitis; dairy cattle; probiotics; prophylactic effect.
© 2022 The Authors. Animal Science Journal published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Animal Science.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bar, D. , Gröhn, Y. T. , Bennett, G. , González, R. N. , Hertl, J. A. , Schulte, H. F. , Tauer, L. W. , Welcome, F. L. , & Schukken, Y. H. (2008). Effects of repeated episodes of generic clinical mastitis on mortality and culling in dairy cows. Journal of Dairy Science, 91(6), 2196–2204. 10.3168/jds.2007-0460 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

