Demonstration of an Optimized Large-scale Optogenetic Cortical Interface for Non-human Primates
- PMID: 36086548
- PMCID: PMC10259873
- DOI: 10.1109/EMBC48229.2022.9871332
Demonstration of an Optimized Large-scale Optogenetic Cortical Interface for Non-human Primates
Abstract
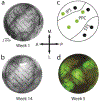
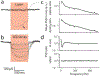
Optogenetics is a powerful neuroscientific tool which allows neurons to be modulated by optical stimulation. Despite widespread optogenetic experimentation in small animal models, optogenetics in non-human primates (NHPs) remains a niche field, particularly at the large scales necessary for multi-regional neural research. We previously published a large-scale, chronic optogenetic cortical interface for NHPs which was successful but came with a number of limitations. In this work, we present an optimized interface which improves upon the stability and scale of our previous interface while using more easily replicable methods to increase our system's availability to the scientific community. Specifically, we (1) demonstrate the long-term (~3 months) optical access to the brain achievable using a commercially-available transparent artificial dura with embedded electrodes, (2) showcase large-scale optogenetic expression achievable with simplified (magnetic resonance-free) surgical techniques, and (3) effectively modulated the expressing areas at large scales (~1 cm2) by light emitting diode (LED) arrays assembled in-house.
Figures

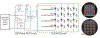
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
