KEAP1-NRF2 protein-protein interaction inhibitors: Design, pharmacological properties and therapeutic potential
- PMID: 36086898
- PMCID: PMC10087726
- DOI: 10.1002/med.21925
KEAP1-NRF2 protein-protein interaction inhibitors: Design, pharmacological properties and therapeutic potential
Abstract
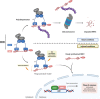
The transcription factor nuclear factor erythroid 2-related factor 2 (NRF2) is considered the master regulator of the phase II antioxidant response. It controls a plethora of cytoprotective genes related to oxidative stress, inflammation, and protein homeostasis, among other processes. Activation of these pathways has been described in numerous pathologies including cancer, cardiovascular, respiratory, renal, digestive, metabolic, autoimmune, and neurodegenerative diseases. Considering the increasing interest of discovering novel NRF2 activators due to its clinical application, initial efforts were devoted to the development of electrophilic drugs able to induce NRF2 nuclear accumulation by targeting its natural repressor protein Kelch-like ECH-associated protein 1 (KEAP1) through covalent modifications on cysteine residues. However, off-target effects of these drugs prompted the development of an innovative strategy, the search of KEAP1-NRF2 protein-protein interaction (PPI) inhibitors. These innovative activators are proposed to target NRF2 in a more selective way, leading to potentially improved drugs with the application for a variety of diseases that are currently under investigation. In this review, we summarize known KEAP1-NRF2 PPI inhibitors to date and the bases of their design highlighting the most important features of their respective interactions. We also discuss the preclinical pharmacological properties described for the most promising compounds.
Keywords: KEAP1-NRF2 protein-protein interaction inhibitors; NRF2; NRF2-ARE pathway therapeutic potential; chronic diseases; phase II antioxidant response.
© 2022 The Authors. Medicinal Research Reviews published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313‐322. - PubMed
-
- Hayes JD, Dinkova‐Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39(4):199‐218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

