Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†
- PMID: 36087287
- PMCID: PMC10248218
- DOI: 10.1093/biolre/ioac173
Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases†
Abstract
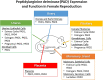
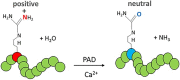
Citrullination, the post-translational modification of arginine residues, is catalyzed by the four catalytically active peptidylarginine deiminase (PAD or PADI) isozymes and alters charge to affect target protein structure and function. PADs were initially characterized in rodent uteri and, since then, have been described in other female tissues including ovaries, breast, and the lactotrope and gonadotrope cells of the anterior pituitary gland. In these tissues and cells, estrogen robustly stimulates PAD expression resulting in changes in levels over the course of the female reproductive cycle. The best-characterized targets for PADs are arginine residues in histone tails, which, when citrullinated, alter chromatin structure and gene expression. Methodological advances have allowed for the identification of tissue-specific citrullinomes, which reveal that PADs citrullinate a wide range of enzymes and structural proteins to alter cell function. In contrast to their important physiological roles, PADs and citrullinated proteins are also involved in several female-specific diseases including autoimmune disorders and reproductive cancers. Herein, we review current knowledge regarding PAD expression and function and highlight the role of protein citrullination in both normal female reproductive tissues and associated diseases.
Keywords: anterior pituitary; citrullination; mammary gland; ovary; peptidylarginine deiminase; uterus.
© The Author(s) 2022. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Our manuscript contains original research, but it is not published nor is it under editorial consideration elsewhere. All research was carried out following ethical guidelines including strict guidelines governing the use of human and animal tissue. None of the authors has anything to disclose.
Figures





References
-
- Rogers GE. Occurrence of citrulline in proteins. Nature 1962; 194:1149–1151. - PubMed
-
- Rogers GE, Harding HW, Llewellyn-Smith IJ. The origin of citrulline-containing proteins in the hair follicle and the chemical nature of trichohyalin, an intracellular precursor. Biochim Biophys Acta 1977; 495:159–175. - PubMed
-
- Rogers GE, Taylor LD. The enzymic derivation of citrulline residues from arginine residues in situ during the biosynthesis of hair proteins that are cross-linked by isopeptide bonds. Adv Exp Med Biol 1977; 86A:283–294. - PubMed
-
- Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays 2003; 25:1106–1118. - PubMed
-
- Chavanas S, Mechin MC, Takahara H, Kawada A, Nachat R, Serre G, Simon M. Comparative analysis of the mouse and human peptidylarginine deiminase gene clusters reveals highly conserved non-coding segments and a new human gene, PADI6. Gene 2004; 330:19–27. - PubMed

