Heart failure outcomes according to heart rate and effects of empagliflozin in patients of the EMPEROR-Preserved trial
- PMID: 36087309
- PMCID: PMC9828798
- DOI: 10.1002/ejhf.2677
Heart failure outcomes according to heart rate and effects of empagliflozin in patients of the EMPEROR-Preserved trial
Abstract
Aims: Empagliflozin reduces cardiovascular death (CVD) or heart failure hospitalization (HHF) in patients with heart failure and preserved ejection fraction (HFpEF). Treatment effects and safety in relation to resting heart rate (RHR) have not been studied.
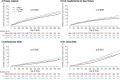
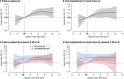
Methods and results: The interplay of RHR and empagliflozin effects in EMPEROR-Preserved was evaluated. We grouped patients (n = 5988) according to their baseline RHR (<70 bpm [n = 2650], 70-75 bpm [n = 967], >75 bpm [n = 1736]) and explored the influence of RHR on CVD or HHF (primary outcome) and its components in sinus rhythm or atrial fibrillation/flutter (AF) and adverse events. We studied the efficacy of empagliflozin across the RHR spectrum. Compared to placebo, empagliflozin did not change heart rate over time. The primary outcome (p for trend = 0.0004) and its components CVD (p trend = 0.0002), first HHF (p for trend = 0.0099) and all-cause death (p < 0.0001) increased with RHR only in sinus rhythm but not AF. The risk increase with RHR was similar in patients with heart failure and mildly reduced ejection fraction (left ventricular ejection fraction [LVEF] 40-49%) and HFpEF (LVEF ≥50%). Baseline RHR had no influence on the effect of empagliflozin on the primary outcomes (p for trend = 0.20), first HHF (p for trend = 0.49). There were no clinically relevant differences in adverse events between empagliflozin and placebo across the RHR groups.
Conclusion: Resting heart rate associates with outcomes only in sinus rhythm but not in AF. Empagliflozin reduced outcomes over the entire RHR spectrum without increase of adverse events.
Keywords: Atrial fibrillation; Cardiovascular outcomes; Empagliflozin; Heart failure; Resting heart rate.
© 2022 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures



References
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. - PubMed
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263–421. - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. - PubMed
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. - PubMed
-
- Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost‐Brama A, et al.; SHIFT Investigators . Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo‐controlled trial. Lancet. 2010;376:886–994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

