Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial
- PMID: 36087611
- PMCID: PMC9451499
- DOI: 10.1016/S2213-2600(22)00297-1
Anti-C5a antibody (vilobelimab) therapy for critically ill, invasively mechanically ventilated patients with COVID-19 (PANAMO): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial
Abstract
Background: Vilobelimab, an anti-C5a monoclonal antibody, was shown to be safe in a phase 2 trial of invasively mechanically ventilated patients with COVID-19. Here, we aimed to determine whether vilobelimab in addition to standard of care improves survival outcomes in this patient population.
Methods: This randomised, double-blind, placebo-controlled, multicentre phase 3 trial was performed at 46 hospitals in the Netherlands, Germany, France, Belgium, Russia, Brazil, Peru, Mexico, and South Africa. Participants aged 18 years or older who were receiving invasive mechanical ventilation, but not more than 48 h after intubation at time of first infusion, had a PaO2/FiO2 ratio of 60-200 mm Hg, and a confirmed SARS-CoV-2 infection with any variant in the past 14 days were eligible for this study. Eligible patients were randomly assigned (1:1) to receive standard of care and vilobelimab at a dose of 800 mg intravenously for a maximum of six doses (days 1, 2, 4, 8, 15, and 22) or standard of care and a matching placebo using permuted block randomisation. Treatment was not continued after hospital discharge. Participants, caregivers, and assessors were masked to group assignment. The primary outcome was defined as all-cause mortality at 28 days in the full analysis set (defined as all randomly assigned participants regardless of whether a patient started treatment, excluding patients randomly assigned in error) and measured using Kaplan-Meier analysis. Safety analyses included all patients who had received at least one infusion of either vilobelimab or placebo. This study is registered with ClinicalTrials.gov, NCT04333420.
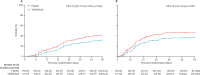
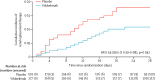
Findings: From Oct 1, 2020, to Oct 4, 2021, we included 368 patients in the ITT analysis (full analysis set; 177 in the vilobelimab group and 191 in the placebo group). One patient in the vilobelimab group was excluded from the primary analysis due to random assignment in error without treatment. At least one dose of study treatment was given to 364 (99%) patients (safety analysis set). 54 patients (31%) of 177 in the vilobelimab group and 77 patients (40%) of 191 in the placebo group died in the first 28 days. The all-cause mortality rate at 28 days was 32% (95% CI 25-39) in the vilobelimab group and 42% (35-49) in the placebo group (hazard ratio 0·73, 95% CI 0·50-1·06; p=0·094). In the predefined analysis without site-stratification, vilobelimab significantly reduced all-cause mortality at 28 days (HR 0·67, 95% CI 0·48-0·96; p=0·027). The most common TEAEs were acute kidney injury (35 [20%] of 175 in the vilobelimab group vs 40 [21%] of 189 in the placebo), pneumonia (38 [22%] vs 26 [14%]), and septic shock (24 [14%] vs 31 [16%]). Serious treatment-emergent adverse events were reported in 103 (59%) of 175 patients in the vilobelimab group versus 120 (63%) of 189 in the placebo group.
Interpretation: In addition to standard of care, vilobelimab improves survival of invasive mechanically ventilated patients with COVID-19 and leads to a significant decrease in mortality. Vilobelimab could be considered as an additional therapy for patients in this setting and further research is needed on the role of vilobelimab and C5a in other acute respiratory distress syndrome-causing viral infections.
Funding: InflaRx and the German Federal Government.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NCR and RG are founders, active officers, and executive directors of the board, and hold shares and stock options in InflaRx. KP is the chief clinical development officer and holds stock options in InflaRx. RZ is the program director and holds stock options in InflaRx. SR is an employee of Metronomia Clinical Research and a contracted statistical service provider for InflaRx. MW receives grants from the German Research Foundation (SFB-TR84 C6, SFB-TR84 C9, and SFB 1449 B02) and German Ministry of Education and Research (e:Med CAPSyS [01ZX1304B], SYMPATH [01ZX1906A], NUM-NAPKON [01KX2021], and PROVID [01KI20160A]). APJV received consulting fees from InflaRx for advisory work, paid to the institution. GM received honoraria for lecturing and consulting from B Braun, 4TEEN4, Adrenomed, and Philips. All other authors declare no competing interests.
Figures


Comment in
-
Complement C5a inhibition: a new form of COVID-19 treatment for mechanically ventilated patients?Lancet Respir Med. 2022 Dec;10(12):1103-1104. doi: 10.1016/S2213-2600(22)00365-4. Epub 2022 Sep 12. Lancet Respir Med. 2022. PMID: 36108660 Free PMC article. No abstract available.
-
C5a-C5aR1 Axis Blockade in Patients With Severe COVID-19: Differences Between PANAMO and FORCE Study.Crit Care Med. 2023 Nov 1;51(11):e243-e244. doi: 10.1097/CCM.0000000000005956. Epub 2023 Oct 12. Crit Care Med. 2023. PMID: 37902353 No abstract available.
References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

