Housing-temperature reveals energy intake counter-balances energy expenditure in normal-weight, but not diet-induced obese, male mice
- PMID: 36088386
- PMCID: PMC9464191
- DOI: 10.1038/s42003-022-03895-8
Housing-temperature reveals energy intake counter-balances energy expenditure in normal-weight, but not diet-induced obese, male mice
Abstract
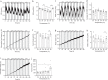
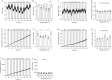
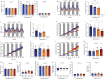
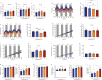
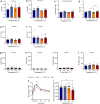
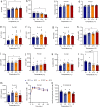
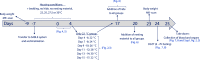
Most metabolic studies on mice are performed at room temperature, although under these conditions mice, unlike humans, spend considerable energy to maintain core temperature. Here, we characterize the impact of housing temperature on energy expenditure (EE), energy homeostasis and plasma concentrations of appetite- and glucoregulatory hormones in normal-weight and diet-induced obese (DIO) C57BL/6J mice fed chow or 45% high-fat-diet, respectively. Mice were housed for 33 days at 22, 25, 27.5, and 30 °C in an indirect-calorimetry-system. We show that energy expenditure increases linearly from 30 °C towards 22 °C and is ~30% higher at 22 °C in both mouse models. In normal-weight mice, food intake counter-balances EE. In contrast, DIO mice do not reduce food intake when EE is lowered. By end of study, mice at 30 °C, therefore, had higher body weight, fat mass and plasma glycerol and triglycerides than mice at 22 °C. Dysregulated counterbalancing in DIO mice may result from increased pleasure-based eating.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing interest: all authors are employed by Novo Nordisk (Denmark), and some are minor shareholders of Novo Nordisk stocks. All authors declare no competing interests that may be of relevance to this work.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources

