Peripheral CB1 receptor blockade acts as a memory enhancer through a noradrenergic mechanism
- PMID: 36088492
- PMCID: PMC9750989
- DOI: 10.1038/s41386-022-01436-9
Peripheral CB1 receptor blockade acts as a memory enhancer through a noradrenergic mechanism
Abstract
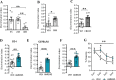
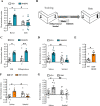
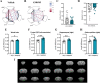
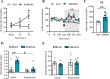
Peripheral inputs continuously shape brain function and can influence memory acquisition, but the underlying mechanisms have not been fully understood. Cannabinoid type-1 receptor (CB1R) is a well-recognized player in memory performance, and its systemic modulation significantly influences memory function. By assessing low arousal/non-emotional recognition memory in mice, we found a relevant role of peripheral CB1R in memory persistence. Indeed, the peripherally-restricted CB1R specific antagonist AM6545 showed significant mnemonic effects that were occluded in adrenalectomized mice, and after peripheral adrenergic blockade. AM6545 also transiently impaired contextual fear memory extinction. Vagus nerve chemogenetic inhibition reduced AM6545-induced mnemonic effect. Genetic CB1R deletion in dopamine β-hydroxylase-expressing cells enhanced recognition memory persistence. These observations support a role of peripheral CB1R modulating adrenergic tone relevant for cognition. Furthermore, AM6545 acutely improved brain connectivity and enhanced extracellular hippocampal norepinephrine. In agreement, intra-hippocampal β-adrenergic blockade prevented AM6545 mnemonic effects. Altogether, we disclose a novel CB1R-dependent peripheral mechanism with implications relevant for lengthening the duration of non-emotional memory.
© 2022. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

