Multiple Vertebral Fractures After Denosumab Discontinuation: FREEDOM and FREEDOM Extension Trials Additional Post Hoc Analyses
- PMID: 36088628
- PMCID: PMC10092421
- DOI: 10.1002/jbmr.4705
Multiple Vertebral Fractures After Denosumab Discontinuation: FREEDOM and FREEDOM Extension Trials Additional Post Hoc Analyses
Abstract
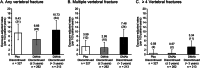
It is uncertain whether the risk of vertebral fracture (VF) and multiple vertebral fractures (MVFs; ≥2 VFs) after denosumab (DMAb) discontinuation is related to treatment duration. A prior analysis of Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) and FREEDOM Extension trials did not find a relationship with DMAb duration and may have underreported MVF incidence because it included women who did not have radiographs. In this post hoc exploratory analysis, the crude incidence and annualized rates of VF and MVF were determined in patients with ≥7 months' follow-up and ≥1 spine radiograph after discontinuing placebo or DMAb. A multivariate analysis was performed to identify predictors of MVF. Clinical characteristics of patients with ≥4 VFs were explored. This analysis included women who discontinued after placebo (n = 327) or DMAb either from FREEDOM or FREEDOM Extension (n = 425). The DMAb discontinuation group was subsequently dichotomized by treatment duration: short-term (≤3 years; n = 262) and long-term (>3 years; n = 213) treatment. For any VF, exposure-adjusted annualized rates per 100 patient-years (95% confidence interval [CI]) were 9.4 (95% CI, 6.4-13.4) for placebo, 6.7 (95% CI, 4.2-10.1) for short-term DMAb, and 10.7 (95% CI, 7.4-15) for long-term DMAb. Annualized rates for MVF were 3.6 (95% CI, 1.9-6.3), 2.9 (95% CI, 1.4-5.4), and 7.5 (95% CI, 4.8-11.1), respectively. Annualized rates for ≥4 VFs were 0.59 (95% CI, 0.1-2.1), 0.57 (95% CI, 0.1-2.1), and 3.34 (95% CI, 1.7-6.0), respectively. In a multivariate regression model, DMAb duration was significantly associated with MVF risk (odds ratio 3.0; 95% CI, 1.4-6.5). Of 15 patients with ≥4 VFs, 13 had DMAb exposure (mean ± standard deviation [SD], 4.9 ± 2.2 years). The risk of MVF after DMAb discontinuation increases with increased duration of DMAb treatment. Patients transitioning off DMAb after 3 years may warrant more frequent administration of zoledronic acid or another bisphosphonate to maintain bone turnover and bone mineral density (BMD) and prevent MVF. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANTIRESORPTIVES; CLINICAL TRIALS; FRACTURE PREVENTION; OSTEOPOROSIS; THERAPEUTICS.
© 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Conflict of interest statement
FC has received grants/research support from Amgen and Radius Health; received consulting fees from Amgen, Radius Health, Enterabio, and Biocon; and has served on speakers' bureaus for Amgen and Radius Health. SH and MMcD are employees and stockholders of Amgen. SRC has received grants/research support and consulting fees from Amgen.
Figures

References
-
- Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long‐term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222‐229. - PubMed
-
- Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96(4):972‐980. - PubMed
-
- Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long‐term denosumab treatment: a post FREEDOM study. Osteoporos Int. 2018;29(1):41‐47. - PubMed
-
- Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long‐term denosumab treatment for osteoporosis. Calcif Tissue Int. 2018;103(1):50‐54. - PubMed
-
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo‐controlled FREEDOM trial and its extension. J Bone Miner Res. 2018;33(2):190‐198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

