Cas12a-assisted RTF-EXPAR for accurate, rapid and simple detection of SARS-CoV-2 RNA
- PMID: 36088673
- PMCID: PMC9444317
- DOI: 10.1016/j.bios.2022.114683
Cas12a-assisted RTF-EXPAR for accurate, rapid and simple detection of SARS-CoV-2 RNA
Abstract
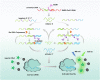
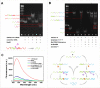
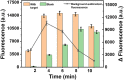
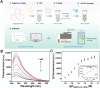
Developing highly accurate and simple approaches to rapidly identify and isolate SARS-CoV-2 infected patients is important for the control of the COVID-19 pandemic. We, herein, reported the performance of a Cas12a-assisted RTF-EXPAR strategy for the identification of SARS-CoV-2 RNA. This assay combined the advantages of RTF-EXPAR with CRISPR-Cas12a can detect SARS-CoV-2 within 40 min, requiring only isothermal control. Particularly, the simultaneous use of EXPAR amplification and CRISPR improved the detection sensitivity, thereby realizing ultrasensitive SARS-CoV-2 RNA detection with a detection limit of 3.77 aM (∼2 copies/μL) in an end-point fluorescence read-out fashion, and at 4.81 aM (∼3 copies/μL) level via a smartphone-assisted analysis system (RGB analysis). Moreover, Cas12a increases the specificity by intrinsic sequence-specific template recognition. Overall, this method is fast, sensitive, and accurate, needing minimal equipment, which holds great promise to meet the requirements of point-of-care molecular detection of SARS-CoV-2.
Keywords: CRISPR-Cas12a; End-point fluorescence; Isothermy; RTF-EXPAR; SARS-CoV-2 RNA.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Ultrarapid detection of SARS-CoV-2 RNA using a reverse transcription-free exponential amplification reaction, RTF-EXPAR.Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2100347118. doi: 10.1073/pnas.2100347118. Proc Natl Acad Sci U S A. 2021. PMID: 34400545 Free PMC article.
-
Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection.Biosens Bioelectron. 2020 Dec 1;169:112642. doi: 10.1016/j.bios.2020.112642. Epub 2020 Sep 20. Biosens Bioelectron. 2020. PMID: 32979593 Free PMC article.
-
Sensitive and visual detection of SARS-CoV-2 using RPA-Cas12a one-step assay with ssDNA-modified crRNA.Anal Chim Acta. 2024 Jun 22;1309:342693. doi: 10.1016/j.aca.2024.342693. Epub 2024 May 5. Anal Chim Acta. 2024. PMID: 38772660
-
Toward a next-generation diagnostic tool: A review on emerging isothermal nucleic acid amplification techniques for the detection of SARS-CoV-2 and other infectious viruses.Anal Chim Acta. 2022 May 29;1209:339338. doi: 10.1016/j.aca.2021.339338. Epub 2021 Dec 1. Anal Chim Acta. 2022. PMID: 35569864 Free PMC article. Review.
-
CRISPR-based detection of SARS-CoV-2: A review from sample to result.Biosens Bioelectron. 2021 Apr 15;178:113012. doi: 10.1016/j.bios.2021.113012. Epub 2021 Jan 21. Biosens Bioelectron. 2021. PMID: 33497879 Free PMC article. Review.
Cited by
-
Robust Sequence Design Space for the Isothermal Exponential Amplification of Short Oligonucleotides.Small. 2024 Nov;20(47):e2405250. doi: 10.1002/smll.202405250. Epub 2024 Aug 24. Small. 2024. PMID: 39180448 Free PMC article.
-
Research Progress on Signal Conversion Based on Aptamer Combined CRISPR/Cas System in Biosensors.Mol Diagn Ther. 2025 Jul;29(4):499-518. doi: 10.1007/s40291-025-00785-7. Epub 2025 Jun 18. Mol Diagn Ther. 2025. PMID: 40531391 Review.
-
Smartphone-based point-of-care testing of the SARS-CoV-2: A systematic review.Sci Afr. 2023 Sep;21:e01757. doi: 10.1016/j.sciaf.2023.e01757. Epub 2023 Jun 10. Sci Afr. 2023. PMID: 37351482 Free PMC article.
-
Three-way junction structure-mediated reverse transcription-free exponential amplification reaction for pathogen RNA detection.Anal Bioanal Chem. 2024 May;416(13):3161-3171. doi: 10.1007/s00216-024-05264-2. Epub 2024 Apr 1. Anal Bioanal Chem. 2024. PMID: 38558309
-
A protospacer adjacent motif-free, multiplexed, and quantitative nucleic acid detection platform with barcode-based Cas12a activity.MedComm (2020). 2023 Jul 2;4(4):e310. doi: 10.1002/mco2.310. eCollection 2023 Aug. MedComm (2020). 2023. PMID: 37405277 Free PMC article.
References
-
- Anderson E.M., Goodwin E.C., Verma A., Arevalo C.P., Bolton M.J., Weirick M.E., Gouma S., McAllister C.M., Christensen S.R., Weaver J., Hicks P., Manzoni T.B., Oniyide O., Ramage H., Mathew D., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., D'Andrea K., Kuthuru O., Dougherty J., Pattekar A., Kim J., Han N., Apostolidis S.A., Huang A.C., Vella L.A., Kuri-Cervantes L., Pampena M.B., Betts M.R., Wherry E.J., Meyer N.J., Cherry S., Bates P., Rader D.J., Hensley S.E. Cell. 2021;184(7):1858–1864. - PMC - PubMed
-
- Chen J., Zhu D., Huang T., Yang Z., Liu B., Sun M., Chen J.X., Dai Z., Zou X. Anal. Chem. 2021;93(37):12707–12713. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

