Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8+ T cells
- PMID: 36088683
- PMCID: PMC9471490
- DOI: 10.1016/j.ebiom.2022.104253
Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8+ T cells
Abstract
Background: Harnessing CD8+ T cell responses is being explored to achieve HIV remission. Although HIV-specific CD8+ T cells become dysfunctional without treatment, antiretroviral therapy (ART) partially restores their function. However, the extent of this recovery under long-term ART is less understood.
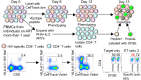
Methods: We analyzed the differentiation status and function of HIV-specific CD8+ T cells after long-term ART initiated in acute or chronic HIV infection ex vivo and upon in vitro recall.
Findings: ART initiation in any stage of acute HIV infection promoted the persistence of long-lived HIV-specific CD8+ T cells with high expansion (P<0·0008) and cytotoxic capacity (P=0·02) after in vitro recall, albeit at low cell number (P=0·003). This superior expansion capacity correlated with stemness (r=0·90, P=0·006), measured by TCF-1 expression, similar to functional HIV-specific CD8+ T cells found in spontaneous controllers. Importanly, TCF-1 expression in these cells was associated with longer time to viral rebound ranging from 13 to 48 days after ART interruption (r =0·71, P=0·03). In contrast, ART initiation in chronic HIV infection led to more differentiated HIV-specific CD8+ T cells lacking stemness properties and exhibiting residual dysfunction upon recall, with reduced proliferation and cytolytic activity.
Interpretation: ART initiation in acute HIV infection preserves functional HIV-specific CD8+ T cells, albeit at numbers too low to control viral rebound post-ART. HIV remission strategies may need to boost HIV-specific CD8+ T cell numbers and induce stem cell-like properties to reverse the residual dysfunction persisting on ART in people treated after acute infection prior to ART release.
Funding: U.S. National Institutes of Health and U.S. Department of Defense.
Keywords: Antiretroviral therapy; CD8 T cells; Cell differentiation; HIV; TCF-1.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests All the other authors declare that they have no competing interests.
Figures






References
-
- Colby DJ, Sarnecki M, Barouch DH, et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat Med. 2020;26(4):498–501. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

