Neuronal circuitry for recognition memory of object and place in rodent models
- PMID: 36089106
- PMCID: PMC10542956
- DOI: 10.1016/j.neubiorev.2022.104855
Neuronal circuitry for recognition memory of object and place in rodent models
Abstract
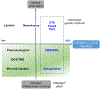
Rats and mice are used for studying neuronal circuits underlying recognition memory due to their ability to spontaneously remember the occurrence of an object, its place and an association of the object and place in a particular environment. A joint employment of lesions, pharmacological interventions, optogenetics and chemogenetics is constantly expanding our knowledge of the neural basis for recognition memory of object, place, and their association. In this review, we summarize current studies on recognition memory in rodents with a focus on the novel object preference, novel location preference and object-in-place paradigms. The evidence suggests that the medial prefrontal cortex- and hippocampus-connected circuits contribute to recognition memory for object and place. Under certain conditions, the striatum, medial septum, amygdala, locus coeruleus and cerebellum are also involved. We propose that the neuronal circuitry for recognition memory of object and place is hierarchically connected and constructed by different cortical (perirhinal, entorhinal and retrosplenial cortices), thalamic (nucleus reuniens, mediodorsal and anterior thalamic nuclei) and primeval (hypothalamus and interpeduncular nucleus) modules interacting with the medial prefrontal cortex and hippocampus.
Keywords: Cell type specificity; Entorhinal cortex; Hippocampus; Medial prefrontal cortex; Object recognition; Spatial memory; Thalamus.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


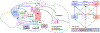
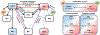
References
-
- Abe H, Ishida Y, Nonaka H, Iwasaki T (2009) Functional difference between rat perirhinal cortex and hippocampus in object and place discrimination tasks. Behav Brain Res 197:388–397. - PubMed
-
- Aggleton JP (1985) One-trial object recognition by rats. Q J Exp Psychol-B 37:279–294.
-
- Aggleton JP (2010) Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia 48:2328–2338. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

