Azithromycin use and outcomes in patients with COVID-19: an observational real-world study
- PMID: 36089152
- PMCID: PMC9458549
- DOI: 10.1016/j.ijid.2022.09.005
Azithromycin use and outcomes in patients with COVID-19: an observational real-world study
Abstract
Objectives: Previous studies ruled out the benefits of azithromycin for treatment of patients with COVID-19 who are hospitalized. However, the effects of azithromycin for treatment of patients with positive SARS-CoV-2 test results in the community remains a matter of debate. This study aimed to assess whether azithromycin, when used in subjects with positive test results for SARS-CoV-2, is associated with a reduced risk of hospitalization, in-hospital COVID-19 outcomes, and death.
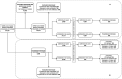
Methods: Two study cohorts were selected. Cohort A included subjects with positive test results for SARS-CoV-2 between February 20, 2020 and December 10, 2020; cohort B included subjects infected with SARS-CoV-2 and hospitalized between February 20, 2020 and December 31, 2020. We compared the risk of hospitalization, intensive care unit access, need for mechanical ventilation, and death in azithromycin users versus nonusers. A clustered Fine-Gray analysis was employed to assess the risk of hospitalization; logistic and Cox regressions were performed to assess the risk of intensive care unit access, mechanical ventilation, and death.
Results: In cohort A, among 4861 azithromycin users and 4861 propensity-matched nonusers, azithromycin use was associated with higher risk of hospitalization (hazard ratio [HR] 1.59, 95% confidence interval [CI] 1.45-1.75) compared with nonuse. In cohort B, among 997 subjects selected in both groups, azithromycin use was not significantly associated with intensive care unit access (odds ratio [OR] 1.22, 95% CI 0.93-1.56), mechanical ventilation (OR 1.30, 95% CI 0.99-1.70), 14-day mortality (HR0.88, 95% CI 0.74-1.05), or 30-day mortality (HR 0.89, 95% CI 0.77-1.03).
Conclusion: Our findings confirm the lack of benefits of azithromycin treatment among community patients infected with SARS-CoV-2, raising concern on potential risks associated with its inappropriate use.
Keywords: Azithromycin; COVID-19; Hospitalization; Intensive care unit access; Italy; Mechanical ventilation; Mortality.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declarations of competing interest ICA, CF, DR, SC, PF, PC, RDP, SK, AZ, GM, AS, GC and GM have no conflicts of interest to declare. LGM reported receiving grants from Bayer, Daiiki-Sankyo, and Boehringer Ingelheim outside the submitted work and speaker fees from Pfizer and Bayer.
Figures


References
-
- Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, Alangaden GJ, Ramesh MS, McKinnon JE, O'Neill W, Zervos M. Henry Ford COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396–403. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

