Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era?
- PMID: 36089157
- PMCID: PMC10229257
- DOI: 10.1016/j.jhep.2022.08.036
Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era?
Abstract
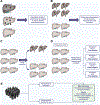
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide, in part because of inadequate early detection strategies. Current recommendations for screening consist of semi-annual abdominal ultrasound with or without serum alpha-fetoprotein in patients with cirrhosis and in demographic subgroups with chronic hepatitis B infection. However, this screening strategy has several deficiencies, including suboptimal early-stage sensitivity, false positives with subsequent harms, inter-operator variability in ultrasound performance, and poor adherence. A blood-based biomarker with sufficient performance characteristics for early-stage disease could overcome several of these barriers to improving early-stage detection. However, prior to use of a biomarker for screening in clinical practice, a multistep validation is required in order to understand test performance characteristics. These steps include case-control validation, followed by validation in prospective cohorts of at-risk patients. Until recently, we lacked adequate longitudinal validation cohorts for early HCC detection; however, several validation cohorts are maturing, including the Hepatocellular Carcinoma Early Detection Study and the Texas Hepatocellular Carcinoma Consortium, which will allow for rigorous validation of candidate biomarkers. While there are several promising biomarkers awaiting validation, in order to supplant abdominal ultrasound, a candidate biomarker must show adequate test performance and overcome practical hurdles to ensure adoption in clinical practice. The promise of blood-based biomarkers is significant, especially given the limitations of ultrasound-based screening; however, they require adequate validation and several logistical obstacles must be overcome prior to clinical implementation.
Keywords: HCC; biospecimen; liver cancer; surveillance.
Copyright © 2022 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Dr. Parikh has served as a consultant for Bristol Myers-Squibb, Exact Sciences, Eli Lilly, and Freenome; has served on advisory boards of Genentech, Eisai, Bayer, Exelixis, Wako/Fujifilm; and has received research funding from Bayer-supportTarget RWE, Exact Sciences, Genentech and Glycotest. Dr. Tayob has no conflicts to declare. Dr. Singal has served on advisory boards or as a consultant for Bayer, FujiFilm Medical Sciences, Exact Sciences, Glycotest, and GRAIL.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

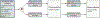
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J Clinicians 2021;71:209–249. - PubMed
-
- Zhang X, El-Serag HB, Thrift AP. Predictors of five-year survival among patients with hepatocellular carcinoma in the United States: an analysis of SEER-Medicare. Cancer Causes & Control 2021;32:317–325. - PubMed
-
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology 2018;68:723–750. - PubMed
-
- Liver EAFTSOT. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

