Characteristics and Outcomes of Suspected Digoxin Toxicity and Immune Fab Treatment Over the Past Two Decades-2000-2020
- PMID: 36089419
- PMCID: PMC9588603
- DOI: 10.1016/j.amjcard.2022.08.004
Characteristics and Outcomes of Suspected Digoxin Toxicity and Immune Fab Treatment Over the Past Two Decades-2000-2020
Abstract
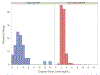
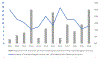
The role of digoxin in clinical practice has narrowed over time. Data on digoxin toxicity trends and outcomes are variable and lack granularity for treatment outcomes. This study aimed to address data gaps in digoxin toxicity trends and outcomes in patients treated with or without digoxin immune fab (DIF). This single-center analysis examined patients with signs/symptoms concerning digoxin toxicity, defined as hospital admission or emergency department visit with elevated digoxin serum concentrations (>2 ng/ml) and/or a primary diagnosis code of digoxin toxicity and/or DIF order. Between 2000 and 2020, 727 patients were identified with signs concerning for digoxin toxicity with a mortality rate of 12.7% during admission and 42.7% at 1 year. DIF was ordered in 9% of cases. Incidence of digoxin toxicity per 1,000 patients with a digoxin prescription and frequency of DIF treatment fluctuated over time without a clear trend toward increase or reduction. DIF-treated patients demonstrated a heavier co-morbidity burden and lower presenting heart rates (median 53 [39.5 to 69.5] vs 77 [64.0 to 91.5] beats/min, p <0.001), worse renal function (median estimated glomerular filtration rate, 30.3 [14.8 to 48.6] vs 40.0 [24.2 to 61.2] ml/min/1.73 m2, p = 0.013), and higher potassium (median 4.5 [4.0 to 5.3] vs 4.3 [3.9 to 4.8] mEq/L, p = 0.022). Compared with a matched cohort, DIF-treated patients experienced a nonsignificant, numerically lower in-hospital mortality (8.2% vs 15.8%, p = 0.199) and 30-day all-cause hospitalization (14.3% vs 24.7%, p = 0.112) and similar 6-month and 1-year hospitalization and mortality. In conclusion, digoxin toxicity remains a pertinent public health issue despite reduction in digoxin utilization. DIF therapy is used in a medically complex population with a high-acuity illness at presentation and is associated with nonsignificant trends toward reduced in-hospital mortality and early readmission that are attenuated over time.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures Dr. Ambrosy has received relevant research support through grants to his institution from Amarin Pharma, Inc., Abbott, and Novartis; and has received modest reimbursement for travel from Novartis. Dr. Fudim receives consulting fees from Abbott, Audicor, AxonTherapies, Bodyguide, Bodyport, Boston Scientific, CVRx, Daxor, Edwards Lifesciences, Feldschuh Foundation, Fire1, Gradient, Intershunt, NXT Biomedical, Pharmacosmos, PreHealth, Shifamed, Splendo, Vironix, Viscardia, and Zoll. The remaining authors have no conflicts of interest to declare.
Figures
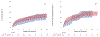
References
-
- Haynes K, Heitjan DF, Kanetsky PA, Hennessy S. Declining public health burden of digoxin toxicity from 1991 to 2004. Clin Pharmacol Ther 2008;84:90–94. - PubMed
-
- Ambrosy AP, Bhatt AS, Stebbins AL, Wruck LM, Fudim M, Greene SJ, Kraus WE, Connor CMO, Piña IL, Whellan DJ, Mentz RJ. Prevalent digoxin use and subsequent risk of death or hospitalization in ambulatory heart failure patients with a reduced ejection fraction — Findings from the Heart Failure : A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION). Am Heart J 2018;199:97–104. - PMC - PubMed
-
- Angraal S, Nuti SV., Masoudi FA, Freeman J V., Murugiah K, Shah ND, Desai NR, Ranasinghe I, Wang Y, Krumholz HM. Digoxin Use and Associated Adverse Events Among Older Adults. Am J Med 2019;132:1191–1198. - PubMed
-
- Kotecha D, Bunting KV, Gill SK, Mehta S, Stanbury M, Jones JC, Haynes S, Calvert MJ, Deeks JJ, Steeds RP, Strauss VY, Rahimi K, Camm AJ, Griffith M, Lip GYH, Townend JN, Kirchhof P, Domingos P, Grant M, Hayes E, Watson H, Sehmi S, Wale R, Slinn G, Jowett S, Mathers J, Stoll V. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: The RATE-AF randomized clinical trial. JAMA - J Am Med Assoc 2020;324:2497–2508. - PMC - PubMed
-
- Hauptman PJ, Blume SW, Lewis EF, Ward S. Digoxin Toxicity and Use of Digoxin Immune Fab. Insights From a National Hospital Database. JACC Hear Fail 2016;4:357–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

