Tao-Hong-Si-Wu decoction improves depressive symptoms in model rats via amelioration of BDNF-CREB-arginase I axis disorders
- PMID: 36089851
- PMCID: PMC9467594
- DOI: 10.1080/13880209.2022.2116460
Tao-Hong-Si-Wu decoction improves depressive symptoms in model rats via amelioration of BDNF-CREB-arginase I axis disorders
Abstract
Context: The traditional Chinese medicine formula Tao-Hong-Si-Wu decoction (TSD), used for treating ischaemic stroke, has the potential to treat depressive disorder (DD).
Objective: To explore the effective targets of TSD on DD animal models.
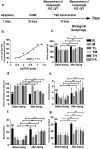
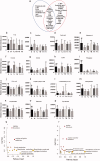
Materials and methods: Sprague-Dawley (SD) rats were modelled by inducing chronic unpredictable mild stress (CUMS) during 35 days and treated with three dosages of TSD (2.5, 5 and 10 g/kg) or fluoxetine (10 mg/kg) by oral gavage for 14 days. Bodyweight measurements and behavioural tests were performed to observe the effect of TSD on the CUMS animals. A gas chromatography coupled with mass spectrometry (GC-MS)-based metabolomic analysis was conducted to reveal the metabolic characteristics related to the curative effect of TSD. Levels of the proteins associated with the feature metabolites were analysed.
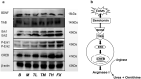
Results: Reduced immobile duration and crossed squares in the behavioural tests were raised by 48.6% and 32.9%, on average, respectively, by TSD treatment (ED50=3.2 g/kg). Antidepressant effects of TSD were associated with 13 decreased metabolites and the restorations of ornithine and urea in the serum. TSD (5 g/kg) raised serum serotonin by 54.1 mg/dL but suppressed arginase I (Arg I) by 47.8 mg/dL in the CUMS rats. Proteins on the brain-derived neurotrophic factor (BDNF)-cAMP response element-binding protein (CREB) axis that modulate the inhibition of Arg I were suppressed in the CUMS rats but reversed by the TSD intervention.
Discussion and conclusions: TSD improves depression-like symptoms in CUMS rats. Further study will focus on the antidepressant-like effects of effective compounds contained in TSD.
Keywords: GC–MS; Metabolomics; depressive disorder; traditional Chinese medicine.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures

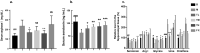
Similar articles
-
Antidepressant-Like Effects and Cognitive Enhancement of Coadministration of Chaihu Shugan San and Fluoxetine: Dependent on the BDNF-ERK-CREB Signaling Pathway in the Hippocampus and Frontal Cortex.Biomed Res Int. 2020 Feb 22;2020:2794263. doi: 10.1155/2020/2794263. eCollection 2020. Biomed Res Int. 2020. PMID: 32185198 Free PMC article.
-
14-3-3ζ Plays a key role in the modulation of neuroplasticity underlying the antidepressant-like effects of Zhi-Zi-Chi-Tang.Phytomedicine. 2023 Jul 25;116:154888. doi: 10.1016/j.phymed.2023.154888. Epub 2023 May 18. Phytomedicine. 2023. PMID: 37257329
-
Mongolian Medicine Areca Thirteen Pill (GY-13) Improved Depressive Syndrome via upregulating cAMP/PKA/CREB/BDNF signaling pathway.J Ethnopharmacol. 2022 Jul 15;293:115310. doi: 10.1016/j.jep.2022.115310. Epub 2022 Apr 20. J Ethnopharmacol. 2022. PMID: 35452773
-
Network pharmacology and experimental evidence: ERK/CREB/BDNF signaling pathway is involved in the antidepressive roles of Kaiyu Zhishen decoction.J Ethnopharmacol. 2024 Jul 15;329:118098. doi: 10.1016/j.jep.2024.118098. Epub 2024 Apr 4. J Ethnopharmacol. 2024. PMID: 38582152
-
Therapeutic potential of plant iridoids in depression: a review.Pharm Biol. 2022 Dec;60(1):2167-2181. doi: 10.1080/13880209.2022.2136206. Pharm Biol. 2022. PMID: 36300881 Free PMC article. Review.
References
-
- Ahmed T, Vasiliadis HM.. 2021. Global cognition modifies the relationship between anemia and depression in old age: a longitudinal analysis of the IMIAS study. Arch Gerontol Geriatr. 94:104342. - PubMed
-
- Chen J, Lin D, Zhang C, Li G, Zhang N, Ruan L, Yan Q, Li J, Yu X, Xie X, et al. . 2015. Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab Brain Dis. 30(1):129–136. - PubMed
-
- Chi X, Wang S, Baloch Z, Zhang H, Li X, Zhang Z, Zhang H, Dong Z, Lu Y, Yu H, et al. . 2019. Research progress on classical traditional Chinese medicine formula Lily Bulb and Rehmannia Decoction in the treatment of depression. Biomed Pharmacother. 112:108616. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
