Genetic identification of tissues and cell types underlying attention-deficit/hyperactivity disorder
- PMID: 36090352
- PMCID: PMC9458853
- DOI: 10.3389/fpsyt.2022.999007
Genetic identification of tissues and cell types underlying attention-deficit/hyperactivity disorder
Abstract
Background: Genome-wide association studies (GWASs) have identified numerous genetic variants associated with attention-deficit/hyperactivity disorder (ADHD), which is considered highly genetically heritable. However, because most of the variants located in the non-coding region of the human genome, the onset of ADHD requires further exploration.
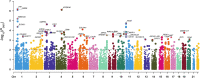
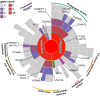
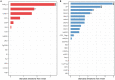
Methods: The risk genes involved in ADHD were identified by integrating GWAS summary data and expression quantitative trait locus (eQTL) data using summary-data-based Mendelian randomization (SMR) method. We then used a stratified linkage disequilibrium score regression (LDSR) method to estimate the contribution of ADHD-relevant tissues to its heritability to screen out disease-relevant tissues. To determine the ADHD-relevant cell types, we used an R package for expression-weighted cell type enrichment (EWCE) analysis.
Results: By integrating the brain eQTL data and ADHD GWAS data using SMR, we identified 247 genes associated with ADHD. The LDSR applied to specifically expressed genes results showed that the ADHD risk genes were mainly enriched in brain tissue, especially in the mesencephalon, visual cortex, and frontal lobe regions. Further cell-type-specific analysis suggested that ADHD risk genes were highly expressed in excitatory neurons.
Conclusion: The study showed that the etiology of ADHD is associated with excitatory neurons in the midbrain, visual cortex, and frontal lobe regions.
Keywords: ADHD; GWAS; cell type enrichment; gene expression; tissues enrichment.
Copyright © 2022 Wei, Sun, Chen, Liu, Zhou, Li and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Kan KJ, Dolan CV, Nivard MG, Middeldorp CM, van Beijsterveldt CE, Willemsen G, et al. Genetic and environmental stability in attention problems across the lifespan: evidence from the Netherlands twin register. J Am Acad Child Adolesc Psychiatry. (2013) 52:12–25. 10.1016/j.jaac.2012.10.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources

