Recent developments in hydrogels containing copper and palladium for the catalytic reduction/degradation of organic pollutants
- PMID: 36090397
- PMCID: PMC9386442
- DOI: 10.1039/d2ra03418b
Recent developments in hydrogels containing copper and palladium for the catalytic reduction/degradation of organic pollutants
Erratum in
-
Correction: Recent developments in hydrogels containing copper and palladium for the catalytic reduction/degradation of organic pollutants.RSC Adv. 2025 Mar 19;15(11):8529. doi: 10.1039/d5ra90028j. eCollection 2025 Mar 17. RSC Adv. 2025. PMID: 40109926 Free PMC article.
Abstract
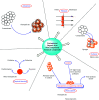
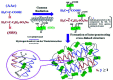
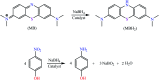
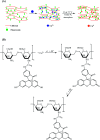
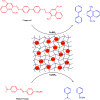
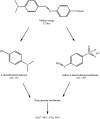
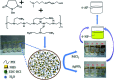
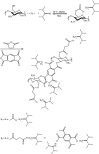
The elimination of toxic and hazardous contaminants from different environmental media has become a global challenge, causing researchers to focus on the treatment of pollutants. Accordingly, the elimination of inorganic and organic pollutants using sustainable, effective, and low-cost heterogeneous catalysts is considered as one of the most essential routes for this aim. Thus, many efforts have been devoted to the synthesis of novel compounds and improving their catalytic performance. Recently, palladium- and copper-based hydrogels have been used as catalysts for reduction, degradation, and decomposition reactions because they have significant features such as high mechanical strength, thermal stability, and high surface area. Herein, we summarize the progress achieved in this field, including the various methods for the synthesis of copper- and palladium-based hydrogel catalysts and their applications for environmental remediation. Moreover, palladium- and copper-based hydrogel catalysts, which have certain advantages, including high catalytic ability, reusability, easy work-up, and simple synthesis, are proposed as a new group of effective catalysts.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



















References
-
- Han F. Kambala V. S. R. Srinivasan M. Rajarathnam D. Naidu R. Appl. Catal., A. 2009;359:25–40.
-
- Tkaczyk J. A. Mitrowska K. Posyniak A. Sci. Total Environ. 2020;717:137222. - PubMed
-
- Zhao O. P. Feng X. Huang D. Yang G. Astruc D. Coord. Chem. Rev. 2015;287:114–136.
-
- Zhang M. Su X. Ma L. Khan A. Wang L. Wang J. Maloletnev A. Yang C. J. Hazard. Mater. 2021;403:123870. - PubMed
-
- Zakaria M. A. Menazea A. Mostafa A. M. Al-Ashkar E. A. Surf. Interfaces. 2020;19:100438.
Publication types
LinkOut - more resources
Full Text Sources

