Halogen-free layered double hydroxide-cyclotriphosphazene carboxylate flame retardants: effects of cyclotriphosphazene di, tetra and hexacarboxylate intercalation on layered double hydroxides against the combustible epoxy resin coated on wood substrates
- PMID: 36090417
- PMCID: PMC9380775
- DOI: 10.1039/d2ra02586h
Halogen-free layered double hydroxide-cyclotriphosphazene carboxylate flame retardants: effects of cyclotriphosphazene di, tetra and hexacarboxylate intercalation on layered double hydroxides against the combustible epoxy resin coated on wood substrates
Abstract
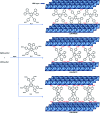
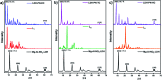
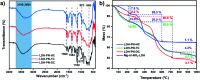
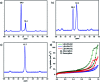
The development of halogen-free flame retardants as environmentally friendly and renewable materials for heat and fire-resistant applications in the field of electronics is important to ensure safety measures. In this regard, we have proposed a simple and halogen-free strategy for the synthesis of flame retardant LDH-PN materials to decrease the fire hazards of epoxy resin (EP), via a co-precipitation reaction between Mg(NO3)2 and Al(NO3)3 and the subsequent incorporation of different cyclotriphosphazene (PN) carboxylate anions. The cyclotriphosphazene-based di, tetra and hexacarboxylate-intercalated layered double hydroxides are designated as LDH-PN-DC, LDH-PN-TC and LDH-PN-HC, respectively. Furthermore, the intercalation of cyclotriphosphazene carboxylate anions into the LDH layers was confirmed by PXRD, FT-IR, TGA, solid-state 31P NMR, nitrogen adsorption and desorption analysis (BET), HR-SEM and XPS. Evaluation of the flame retardant (vertical burning test and limiting oxygen index) properties was demonstrated by formulating the LDH-PN materials with epoxy resin (EP) in different ratios coated on wood substrates to achieve the desired behaviour of the EP/LDH-PN composites. Structure-property analysis reveals that EP/LDH-PN-TC-20 wt% and EP/LDH-PN-HC-20 wt% achieved a V 0 rating in the UL-94 V test and achieved higher LOI values (27.7 vol% for EP/LDH-PN-TC-20 wt% and 29 vol% for EP/LDH-PN-HC-20 wt%) compared to the epoxy-coated wood substrate (23.2 vol%), whereas EP/LDH-PN-DC failed in the vertical burning test for various weight percentages of LDH-PN-DC from 5 wt% to 20 wt% in the composites, with a lower LOI value of 22.1 vol%. Excellent flame retardancy was observed for EP/LDH-PN-TC and EP/LDH-PN-HC due to the presence of more binding sites of carboxylate anions in the LDH layers and less or no spiro groups in cyclotriphosphazene compared to that in EP/LDH-PN-DC. In addition, the synergistic flame retardant effect of the combination of LDH and cyclotriphosphazene on the epoxy resin composites remains very effective in creating a non-volatile protective film on the surface of the wood substrate to shelter it from air, absorb the heat and increase the ignition time, which prevents the supply of oxygen during the combustion process. The results of this study show that the proposed strategy for designing flame-retardant properties represents the state-of-the-art, competent coating of inorganic materials for the protection and functionalization of wood substrates.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
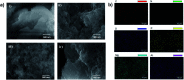
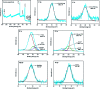



References
-
- Troitzsch J. Flame retardants. Kunstst. Int. 1996;86:960–964.
- Kampke-Thiel K. Lenoir D. Kettrup A. Herdtweck E. Gleich D. Thiel W. R. Isolation, characterization, and toxicological aspects of volatile organophosphorus compounds from the combustion of flame-retarded epoxy resins with phosphonate substructures. Chem.–Eur. J. 1998;4:1581–1586.
- Zhang S. Jiang Y. Sun Y. Sun J. Xu B. Li H. Gu X. Preparation of flame retardant and conductive epoxy resin materials by incorporating functionalized multi-walled carbon nanotubes and graphite sheets. Polym. Adv. Technol. 2021;32:2093–2101.
-
- Venier M. Salamova A. Hites R. A. Halogenated flame retardants in the great lakes environment. Acc. Chem. Res. 2015;48:1853–1861. - PubMed
- Thoma H. Rist S. Hauschulz G. Hutzinger O. Polybrominated dibenzodioxins (PBrDD) and dibenzofurans (PBrDF) in some flame retardant preparations. Chemosphere. 1986;15:2111–2113.
-
- Lenoir D. and Kampke-Thiel K., Formation of polybrominated dibenzodioxins and furans in laboratory combustion processes of brominated flame retardants, in Fire and Polymers, Ed., G. Nelson, ACS Series No. 599, 1995, 2, pp. 377–392
- De Wit C. A. An Overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. - PubMed
-
- Birnbaum L. S. Staskal D. F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004;112:9–17. - PMC - PubMed
- Wei Y.-L. Bao L.-J. Wu C.-C. Zeng E. Y. Characterization of anthropogenic impacts in a large urban center by examining the spatial distribution of halogenated flame retardants. Environ. Pollut. 2016;215:187–194. - PubMed
LinkOut - more resources
Full Text Sources

