Refining Kidney Survival in 383 Genetically Characterized Patients With Nephronophthisis
- PMID: 36090483
- PMCID: PMC9459005
- DOI: 10.1016/j.ekir.2022.05.035
Refining Kidney Survival in 383 Genetically Characterized Patients With Nephronophthisis
Abstract
Introduction: Nephronophthisis (NPH) comprises a group of rare disorders accounting for up to 10% of end-stage kidney disease (ESKD) in children. Prediction of kidney prognosis poses a major challenge. We assessed differences in kidney survival, impact of variant type, and the association of clinical characteristics with declining kidney function.
Methods: Data was obtained from 3 independent sources, namely the network for early onset cystic kidney diseases clinical registry (n = 105), an online survey sent out to the European Reference Network for Rare Kidney Diseases (n = 60), and a literature search (n = 218).
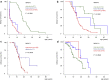
Results: A total of 383 individuals were available for analysis: 116 NPHP1, 101 NPHP3, 81 NPHP4 and 85 NPHP11/TMEM67 patients. Kidney survival differed between the 4 cohorts with a highly variable median age at onset of ESKD as follows: NPHP3, 4.0 years (interquartile range 0.3-12.0); NPHP1, 13.5 years (interquartile range 10.5-16.5); NPHP4, 16.0 years (interquartile range 11.0-25.0); and NPHP11/TMEM67, 19.0 years (interquartile range 8.7-28.0). Kidney survival was significantly associated with the underlying variant type for NPHP1, NPHP3, and NPHP4. Multivariate analysis for the NPHP1 cohort revealed growth retardation (hazard ratio 3.5) and angiotensin-converting enzyme inhibitor (ACEI) treatment (hazard ratio 2.8) as 2 independent factors associated with an earlier onset of ESKD, whereas arterial hypertension was linked to an accelerated glomerular filtration rate (GFR) decline.
Conclusion: The presented data will enable clinicians to better estimate kidney prognosis of distinct patients with NPH and thereby allow personalized counseling.
Keywords: end-stage kidney disease; genetic variant severity; genotype-phenotype correlations; kidney survival; nephronophthisis; prognostic factors.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures




References
-
- König J, Ermisch-Omran B, Omran H. Nephronophthisis and autosomal dominant interstitial kidney disease (ADIKD). Pediatric Kidney Disease. In: Geary, D., Schaefer, F. (eds) Pediatric Kidney Disease. Springer; 2021:369-388.
-
- Titieni A., König J. Nephronophthise und assoziierte Ziliopathien. med genet. 2018;30:461–468. doi: 10.1007/s11825-018-0213-3. - DOI
LinkOut - more resources
Full Text Sources

