Off-label use of cinacalcet in pediatric primary hyperparathyroidism: A French multicenter experience
- PMID: 36090548
- PMCID: PMC9449487
- DOI: 10.3389/fped.2022.926986
Off-label use of cinacalcet in pediatric primary hyperparathyroidism: A French multicenter experience
Abstract
Background: Cinacalcet is a calcimimetic approved in adults with primary hyperparathyroidism (PHPT). Few cases reports described its use in pediatric HPT, with challenges related to the risk of hypocalcemia, increased QT interval and drug interactions. In this study, we report the French experience in this setting.
Methods: We retrospectively analyzed data from 18 pediatric patients from 7 tertiary centers who received cinacalcet for PHPT. The results are presented as median (interquartile range).
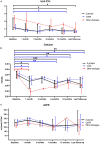
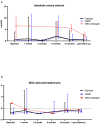
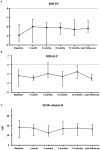
Results: At a median age of 10.8 (2.0-14.4) years, 18 patients received cinacalcet for primary HPT (N = 13 inactive CASR mutation, N = 1 CDC73 mutation, N = 1 multiple endocrine neoplasia type 1, N=3 unknown etiology). Cinacalcet was introduced at an estimated glomerular filtration rate (eGFR) of 120 (111-130) mL/min/1.73 m2, plasma calcium of 3.04 (2.96-3.14) mmol/L, plasma phosphate of 1.1 (1.0-1.3) mmol/L, age-standardized (z score) phosphate of -3.0 (-3.5;-1.9), total ALP of 212 (164-245) UI/L, 25-OHD of 37 (20-46) ng/L, age-standardized (z score) ALP of -2.4 (-3.7;-1.4), PTH of 75 (59-123) ng/L corresponding to 1.2 (1.0-2.3)-time the upper limit for normal (ULN). The starting daily dose of cinacalcet was 0.7 (0.6-1.0) mg/kg, with a maximum dose of 1.0 (0.9-1.4) mg/kg per day. With a follow-up of 2.2 (1.3-4.3) years on cinacalcet therapy, PTH and calcium significantly decreased to 37 (34-54) ng/L, corresponding to 0.8 (0.5-0.8) ULN (p = 0.01), and 2.66 (2.55-2.90) mmol/L (p = 0.002), respectively. In contrast, eGFR, 25-OHD, ALP and phosphate and urinary calcium levels remained stable. Nephrocalcinosis was not reported but one patient displayed nephrolithiasis. Cinacalcet was progressively withdrawn in three patients; no side effects were reported.
Conclusions: Cinacalcet in pediatric HPT can control hypercalcemia and PTH without significant side effects.
Keywords: Calcium-sensing Receptor (CaSR); children; cinacalcet; hypercalcemia; primary hyperparathyroidism.
Copyright © 2022 Bernardor, Flammier, Salles, Amouroux, Castanet, Lienhardt, Martinerie, Damgov, Linglart and Bacchetta.
Conflict of interest statement
JBa is a clinical investigator for industry-sponsored clinical trials on the use of calcimimetics (cinacalcet and etelcalcetide) in pediatric dialysis (Amgen). JBa has received research grants from Amgen (RENOCLASTE study: ID-RCB 2017-A03241-52). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Conte-Devolx B, Niccoli-Sire P. Groupe d'etude des tumeurs à calcitonine. [Polyadenomatoses: type 2 multiple endocrine neoplasms]. Presse Med. (2002) 31:1224–30. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

